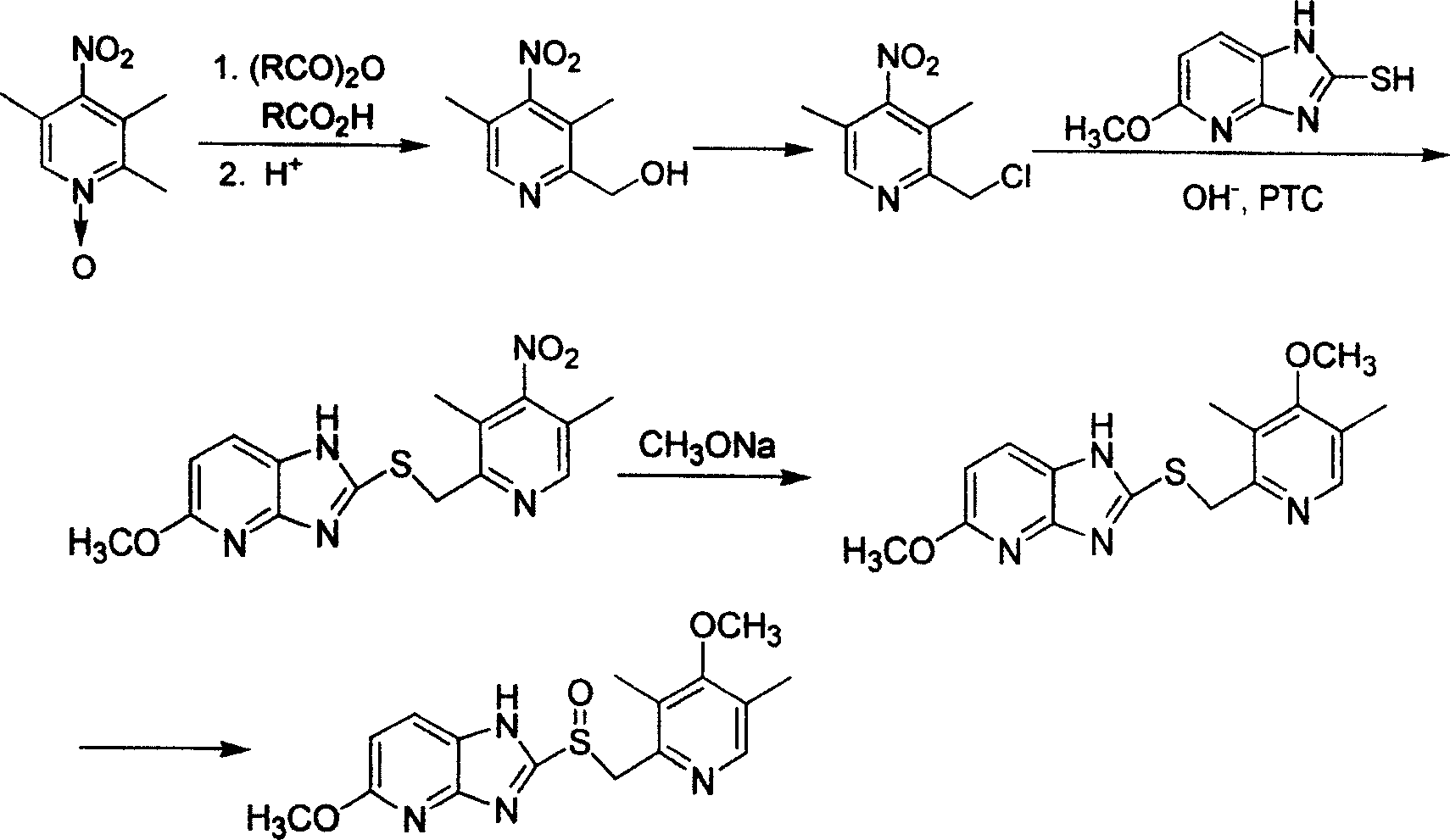
Preparation process of taytrolazole
A technology of tetraprazole and methoxyimidazole, which is applied in the field of novel H+/K+-ATPase inhibitors, can solve the problems of cumbersome preparation process, troublesome post-processing, violent reaction, etc., and achieves easy availability of raw materials and good product purity , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Preparation of 2-hydroxymethyl-3,5-dimethyl-4-nitropyridine
[0022] Mix 50g (0.27mol) of 2,3,5-trimethyl-4-nitropyridine-N-oxide and 40g of glacial acetic acid, stir and heat to 90°C, slowly add 41g (0.40mol) of acetic anhydride dropwise, approximately After 50 minutes of dripping, control the reaction temperature to 90°C, continue the reaction for 2h, after the reaction is complete, recover the solvent under reduced pressure, cool to 70°C, add 150g of hydrochloric acid with a mass concentration of 15%, keep the reaction for 2h, and use the mass percentage for the reaction. The 10% sodium carbonate aqueous solution was neutralized to pH 8, the aqueous layer was extracted with chloroform (100ml×3), the extracts were combined, concentrated, and dried to obtain 39.5g of white powdery solid, yield: 79%, mp: 65.2~66 ℃.
[0023] 2) Preparation of 2-chloromethyl-3,5-dimethyl-4-nitropyridine hydrochloride
[0024] Mix 20g (0.11mol) of 2-hydroxymethyl-3,5-dimethyl-4-nitropyridin...
Embodiment 2
[0032] 1) Preparation of 2-hydroxymethyl-3,5-dimethyl-4-nitropyridine
[0033] Mix 50g (0.27mol) of 2,3,5-trimethyl-4-nitropyridine-N-oxide and 75g of propionic acid, stir and heat to 120°C, slowly add 75g (0.577mol) of propionic anhydride, approximately After 50 minutes of dripping, control the reaction temperature to 120°C, continue the reaction for 1 hour, after the reaction is complete, recover the solvent under reduced pressure, cool to 60°C, add 100 g of hydrochloric acid with a mass percentage of 20%, keep the reaction temperature for 1.5 hours, and use the mass after the reaction is completed A 10% sodium carbonate aqueous solution was neutralized to pH 8, the aqueous layer was extracted with dichloromethane (100ml×3), the extracts were combined, concentrated, and dried to obtain 40.5g of white powdery solid, yield: 81% , Mp: 65.2~66℃.
[0034] 2) Preparation of 2-chloromethyl-3,5-dimethyl-4-nitropyridine hydrochloride
[0035] Mix 20g (0.11mol) of 2-hydroxymethyl-3,5-dime...
Embodiment 3
[0043] 1) Preparation of 2-hydroxymethyl-3,5-dimethyl-4-nitropyridine
[0044] Mix 50g (0.27mol) of 2,3,5-trimethyl-4-nitropyridine-N-oxide and 125g of butyric acid, stir and heat to 90°C, slowly add 100g (0.63mol) of butyric anhydride dropwise, approximately After 1h dripping, control the reaction temperature to 70℃ and continue the reaction for 2.5h. After the reaction is completed, the solvent is recovered under reduced pressure, and the temperature is reduced to 50℃. 280g sulfuric acid with a mass concentration of 10% is added. The concentration of 10% sodium carbonate aqueous solution was neutralized to pH 8, the aqueous layer was extracted with 1,2-dichloroethane (100ml×3), the extracts were combined, concentrated, and dried to obtain 40.7g of white powdery solid. Yield : 81.4%, mp: 65.2~66°C.
[0045] 2) Preparation of 2-chloromethyl-3,5-dimethyl-4-nitropyridine hydrochloride
[0046] Mix 20g (0.11mol) of 2-hydroxymethyl-3,5-dimethyl-4-nitropyridine and 50g of 1,2-dichloroe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com