Protein for promoting erythrocyte growth factor activity and its use
A protein and red blood cell technology, applied in the fields of peptide/protein components, medical preparations containing active ingredients, extracellular fluid diseases, etc., can solve the problem of insufficient amino acids, and achieve the effect of less protein dosage and accurate binding constant measurement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Selection of PH domain mutation sites
[0026]We used the calculation strategy of protein function grafting developed in our laboratory (Biopolymers, 54:515-523, 2000) for the calculation of functional region grafting on the surface of EPO. The key residues at the interaction interface between EPO (Protein Data Bank code leer) and its receptor EPOR: PHE at position 48, ASN at position 147, and ARG at position 150 are used as key residues for grafting. We used a grafting strategy based on vector matching to transfer the key binding site of the protein to another non-homologous protein, and successfully found the candidate protein backbone PH domain for grafting EPO function (Protein Data Bank code is lmai) . Calculated by a computer program we wrote according to this strategy, the complementarity score of this backbone is 561, and the mean square deviation of key residues is 0.843 angstroms. The three key residues that need to be mutated correspond to the fo...
Embodiment 2
[0030] Example 2: Construction, expression and purification of PH domain mutants
[0031] 1. Gene amplification and expression vector construction
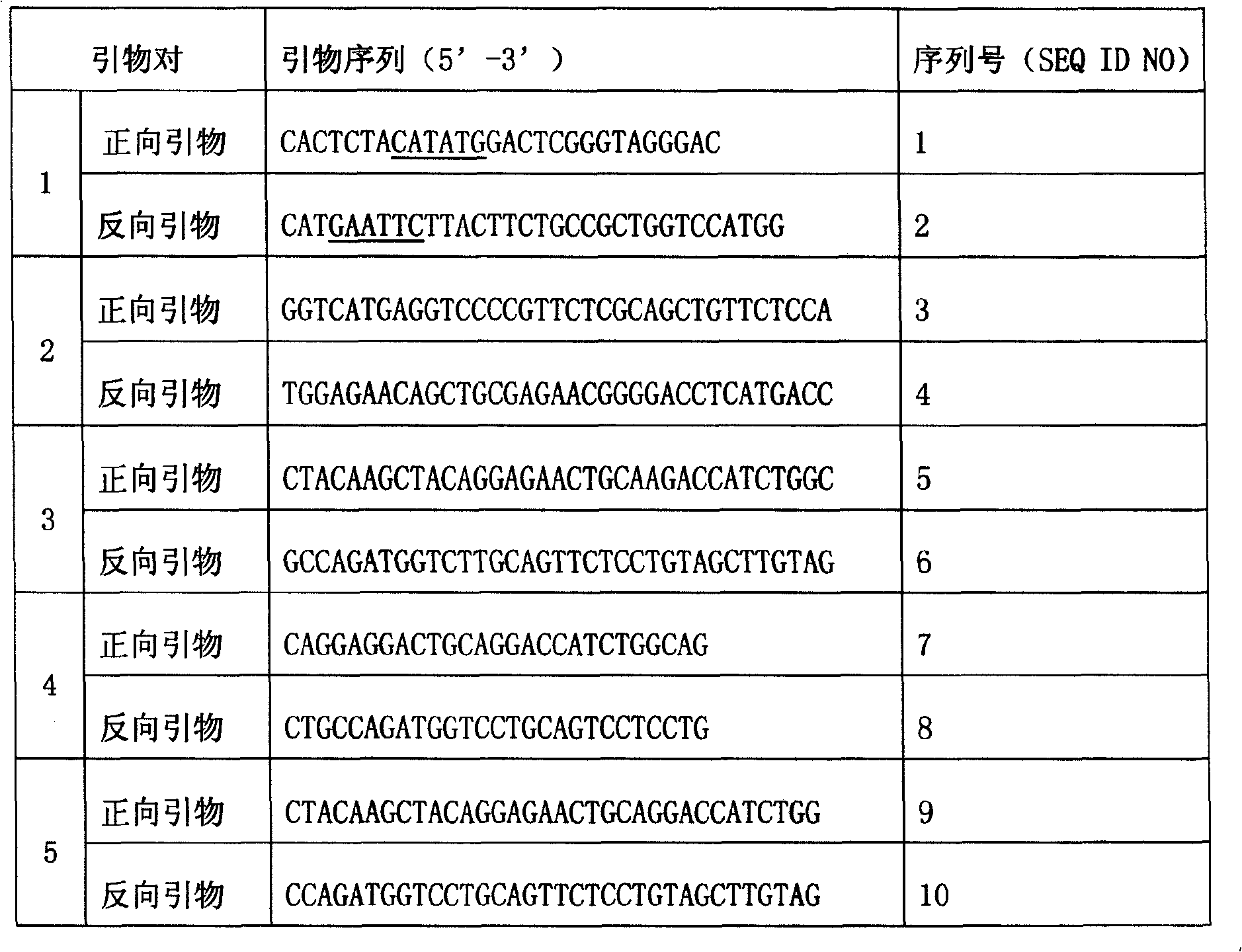
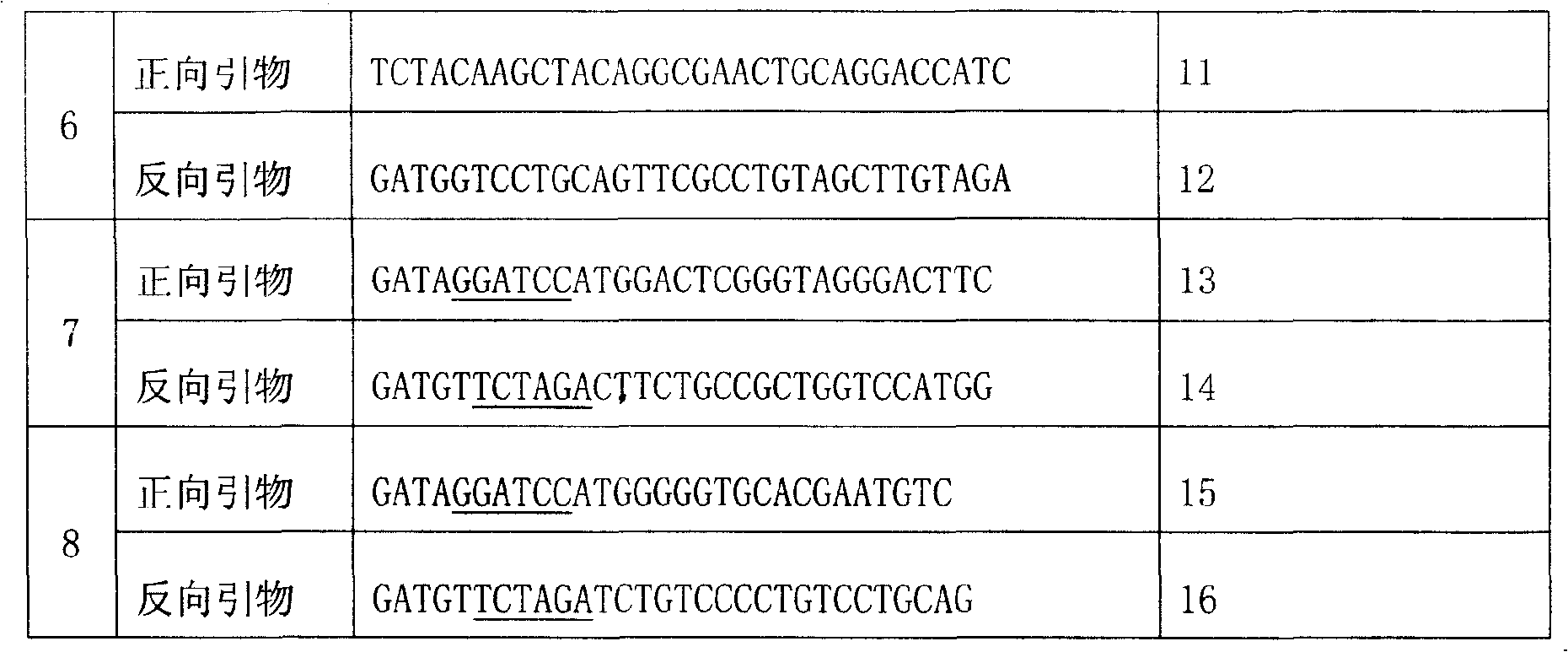
[0032] The gene of the PH domain (residue 1-140; ProteinData Bank code is 1mai) of rat (Rat) phospholipase Cδ1 (Phospholipase C Delta 1) was expressed in pGST4 / PLCδ1 by primer pair 1 (the primer sequence is shown in Table 1) The plasmid (gifted and authorized by Professor Hitoshi Yagisawa; Eur. J. Biochem. 265: 481-490, 1999) was used as a template and amplified by Polymerase Chain Reaction (PCR). After purification, the amplified product was double-digested with NdeI and EcoRI, and the digested product was ligated into the pET-28a vector (purchased from Novagen) that had been double-digested with NdeI and EcoRI by T4 DNA ligase to obtain the expression vector pETPHD. The correct sequence was verified by DNA sequencing.
[0033] Table 1. Primer Design
[0034]
[0035]
[0036] Note: ____ mark is the endonuclease site. C...
Embodiment 3
[0068] Example 3: Detection of receptor binding ability in vitro
[0069]The binding ability of wtNH-PHD and its mutant proteins to EPOR (EPO receptor) was tested by surface plasmon resonance (SPR). The experimental equipment was Biacore 3000 (purchased from Uppsala Company, Sweden), and the recombinant human EPO soluble receptor (rhEPO sR) was purchased from R&D Systems. The buffer used in the experiment was HBS-EP (10 mM Hepes, 150 mM NaCl, 3.7 mM EDTA, pH 7.4, 0.005% P20), and the chip was a CM5 chip.
[0070] rhEPO sR was dissolved in 100 mM sodium acetate solution at pH 3.1 to obtain a solution at pH 4 for immobilization. During the immobilization process, the flow rate was maintained at 5 μL min -1 . Activate the second channel of the CM5 chip with 35 μL N-ethyl-N'-(3-diethylaminopropyl)-carbodiimide / N-hydroxysuccinimide (EDC / NHS, 1:1), then inject 45 μL rhEPO sR, and finally inject 35 μL 1M ethanolamine- HCl, pH 8.5 blocked the channels. The fixed rhEPO sR was 1200...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com