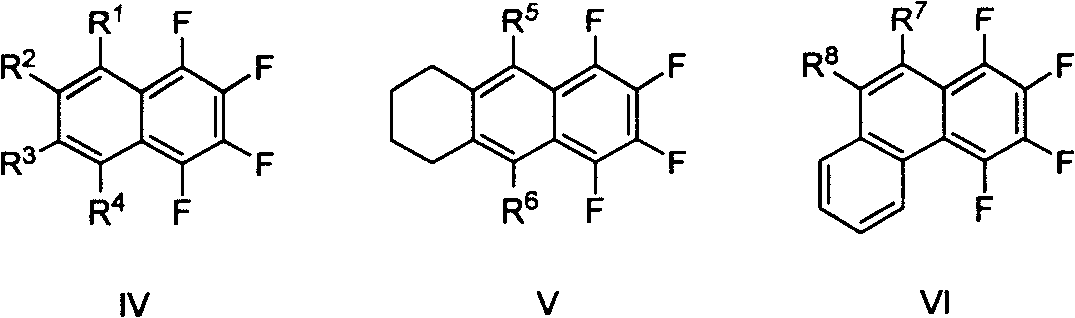
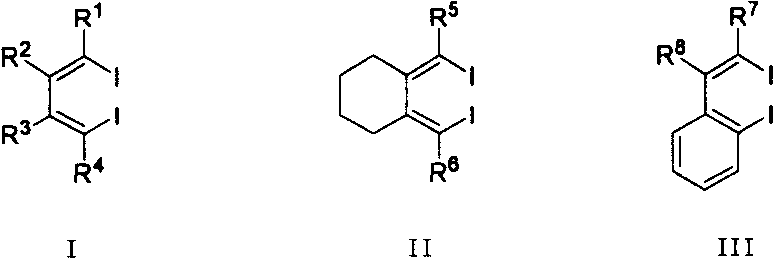
Method for synthesizing multiple fluoro-substituted naphthalene derivative from hexafluorobenzene
A technology of polyfluoronaphthalene derivatives and derivatives, which is applied in the field of synthesis of polyfluoronaphthalene derivatives, and can solve problems such as single substituents of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] One of the general structural formula IV class compounds (R 1 = R 2 = R 3 = R 4 =n-Et): Synthesis of 1,2,3,4-tetraethyl-5,6,7,8-tetrafluoro-naphthalene
[0030] Under the protection of an inert gas (such as high-purity nitrogen), add 1 mmol of 4,5-diethyl-3,6-diiodo-3 , 5-octadiene and 5 mL of anhydrous and oxygen-free treated THF solvent. First, the above THF solution was lowered to a lower temperature (-78°C to -55°C) with a cold bath (such as a dry ice-acetone bath), and then 4 mmol tert-butyllithium (1.6M, n-pentane solution) was added dropwise under magnetic stirring. ). After stirring the reaction at -78°C to -55°C for 1 hour, 1 mmol of hexafluorobenzene and 5 ml of DME were added. The cooling bath was removed, and the reaction solution was gradually warmed to room temperature (20°C to 28°C), stirred for 3 hours, and then washed with about 1 mL of saturated NaHCO 3Aqueous solution quenched the reaction. Extracted three times with ether (10 mL each time). ...
Embodiment 2
[0032] The second (R 1 =R 2 =n-Bu, R 3 =R 4 =Me): Synthesis of 1,2-dimethyl-3,4-dibutyl-5,6,7,8-tetrafluoro-naphthalene
[0033] The synthetic route is basically the same as above. The starting material diiodide compound for this synthesis is 5-(1-methyl-2-iodo-1-propenyl)-6-iodo-5-decene. 0.081 g of pure product was obtained (purity>98%, colorless liquid). Isolated yield 54%. The NMR and high-resolution mass spectrometry data of the compound are as follows. 1 H NMR (400MHz, CDCl 3 ): δ0.98-1.03(m, 6H), 1.51-1.55(m, 8H), 2.39(s, 3H), 2.65(d, J=7.9Hz, 3H), 2.74-2.78(m, 2H), 3.03(br,2H). 13 C NMR (400MHz, CDCl 3 ( m), 145.37(m). HRMS: calcd for C 20 h 24 f 4 340.1814, found 340.1814.
Embodiment 3
[0035] Three (R 1 =R 3 = Ph, R 2 =R 4 =n-Bu): Synthesis of 1,3-dibutyl-2,4-diphenyl-5,6,7,8-tetrafluoro-naphthalene
[0036] The synthetic route is basically the same as above. The starting material diiodide compound for this synthesis is 6-phenyl-7-(1-phenyl-1-iodo-methylene)-5-iodo-5-undecene. 0.311 g of pure product was obtained (purity>98%, colorless solid, melting point 73-74°C). Isolated yield 67%. The NMR, high-resolution mass spectrometry and elemental analysis data of the compound are as follows. 1 H NMR (400MHz, CDCl 3 ): δ0.38(t, J=7.3Hz, 3H), 0.67-0.76(m, 5H), 1.05-1.12(m, 2H), 1.18-1.24(m, 2H), 1.46-1.51(m, 2H ), 2.06-2.10(m, 2H), 2.77-2.82(m, 2H), 7.22-7.27(m, 4H), 7.36-7.45(m, 6H). 13 C NMR (400MHz, CDCl 3 ): δ12.98, 13.54, 22.72, 23.08, 31.23, 32.63(m), 34.11, 117.91(m), 119.76, 126.94, 127.19, 127.63, 128.01, 129.14, 129.17, 129.65, 132.602, 135.37 , 139.66(m), 139.94, 140.87, 142.10(m), 142.80, 144.61(m), 145.40(m). HRMS: calcd for C 30 h 28 f 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com