Chitin sulfuric-ester hydroxy-benzene disulfonic acid derivative and production thereof
A technology of hydroxybenzene disulfonamide and chitosan sulfate, applied in the field of marine chemical engineering, can solve problems such as no relevant reports, and achieve the effects of expanding the application field, good water solubility, and enhancing biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1g of chitosan C-6 sulfate with a molecular weight of 160,000 is dissolved in 50mL of 2% HAc, wherein the chitosan C-6 sulfate is referred to Concepts for improved regioselective placement of O-suifo, N-sulfo, N-acetyl , and N-carboxymethyl groups inchitosan derivatives; Hanno Baumann, Volker Faust; CarbohydrateResearch, 2001, 331, 43-57. The known method is prepared; under stirring, add 1.8g2-hydroxyl-5-chloro-1,3-benzenedi Sulfonyl chloride, react at 65°C for 5 hours. After cooling to room temperature, pour the reaction mixture into 300mL of acetone to make it completely precipitated. To obtain the precipitate, do not use too much acetone. After the precipitate is filtered, it is washed with acetone and dried at 50°C to obtain a brown powder. Use cyclohexane as the solvent After Soxhlet extraction for 8 hours, the hydroxybenzenedisulfonamide derivative of chitosan was obtained by vacuum drying. The structural formula is shown in formula 1, wherein n=496.
[0031] Inf...
Embodiment 2
[0033] The difference from Example 1 is:
[0034] 2g of chitosan C-6 sulfuric acid ester with a molecular weight of 04,000 was dissolved in 50mL of 1% HAc, and 3.45g of 2-hydroxy-5-chloro-1,3-benzenedisulfonyl chloride was added under stirring, and reacted at 75°C for 4 hours. After cooling, pour the reaction mixture into 400 mL of absolute ethanol to obtain a precipitate, filter the precipitate, wash with absolute ethanol, dry at 55°C to obtain a brown powder, use cyclohexane as a solvent for Soxhlet extraction for 8 hours, and then vacuum-dry to obtain the shell Hydroxybenzenedisulfonamide derivatives of polysaccharide sulfate esters, see formula 1 for the structural formula, wherein n=12.
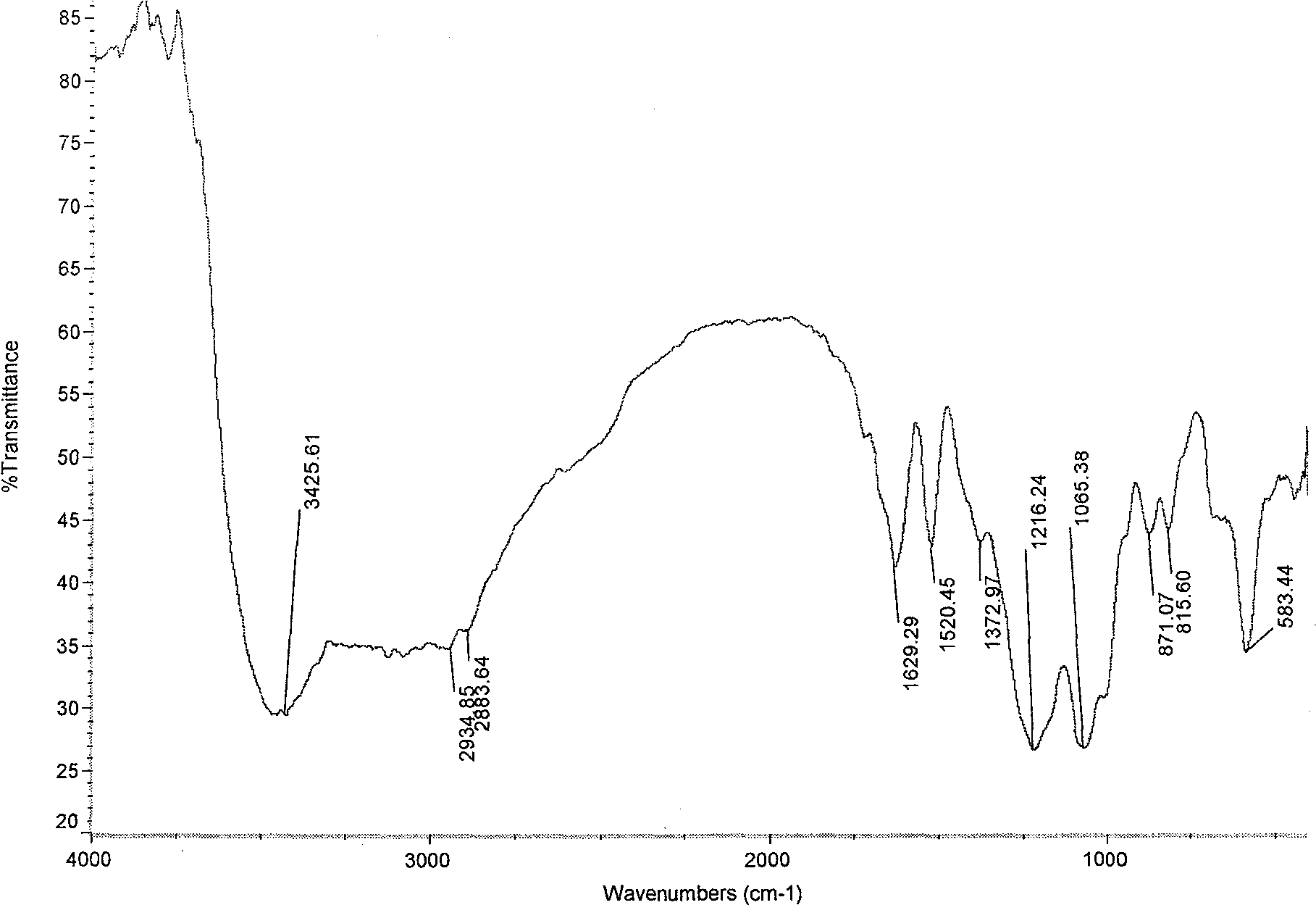
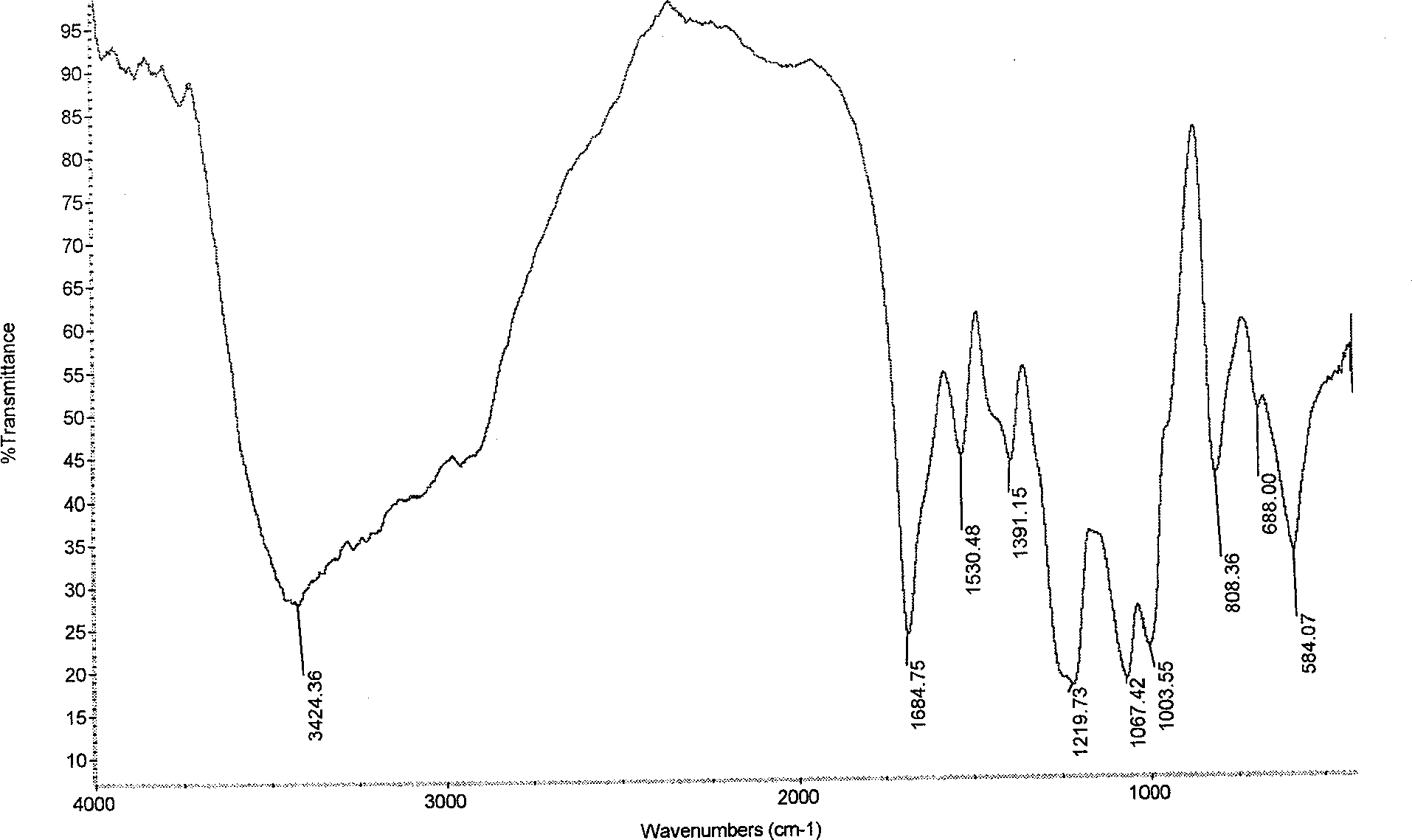
[0035] Infrared spectroscopy analysis shows that the hydroxybenzenedisulfonamide derivatives of chitosan sulfate (see image 3 ) and chitosan sulfate (see figure 1), two new absorption peaks appear at 1530.48 and 1391.15, which are the characteristic absorption peaks of the hydroxybenz...
Embodiment 3
[0037] The difference from Example 1 is:
[0038] 2g of chitosan C-6 sulfate ester with a molecular weight of 50,000 was dissolved in 60mL of 1% HAc, and 2.25g of 2-hydroxy-5-chloro-1,3-benzenedisulfonyl chloride was added under stirring, and reacted at 65°C for 5 hours. After cooling, pour the reaction mixture into 300 mL of absolute ethanol to obtain a precipitate, filter the precipitate, wash with absolute ethanol, dry at 60°C to obtain a brown powder, use cyclohexane as a solvent for Soxhlet extraction for 8 hours, and then vacuum-dry to obtain the shell Hydroxybenzenedisulfonamide derivatives of polysaccharide sulfate esters, see formula 1 for the structural formula, wherein n=155.
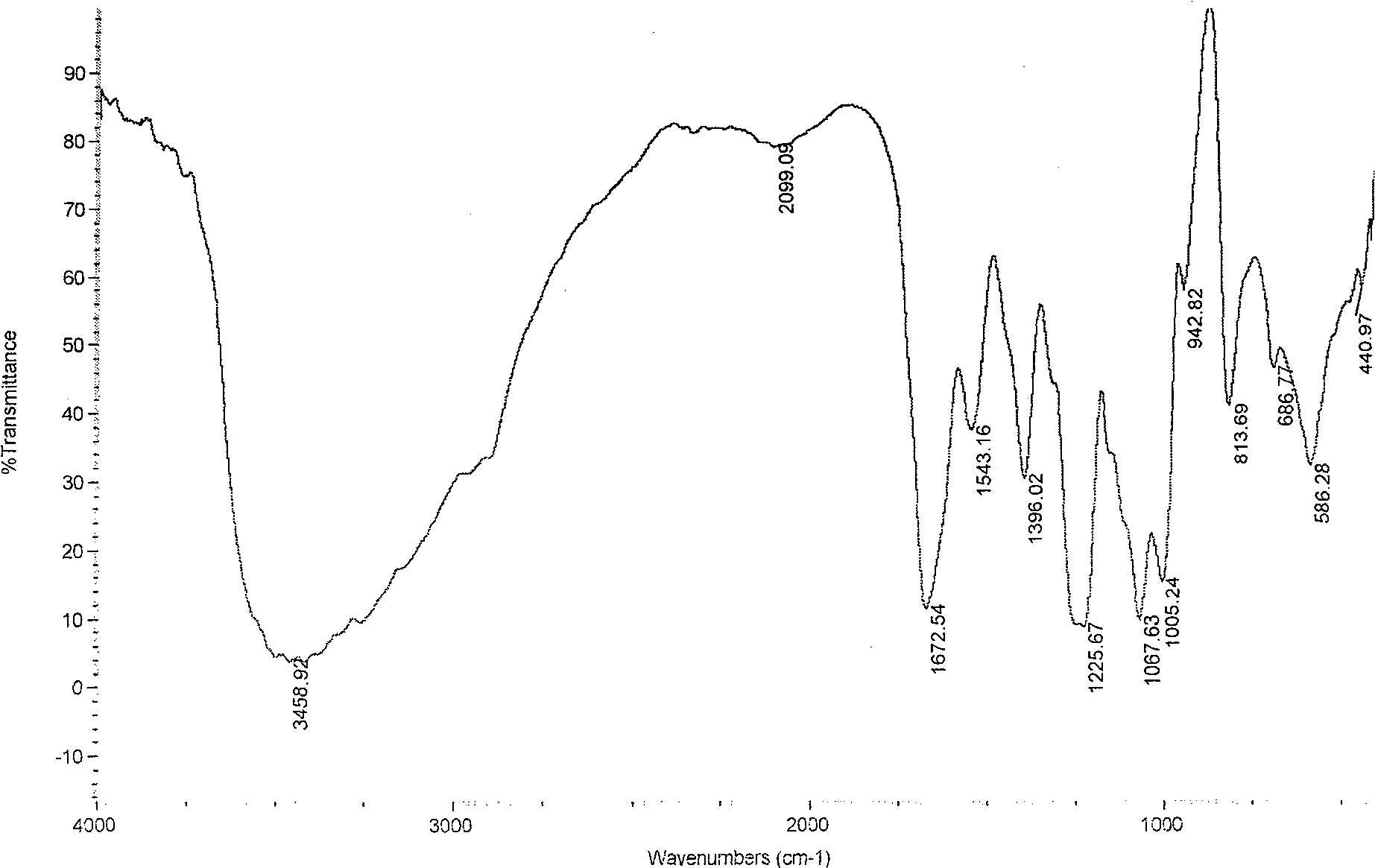
[0039] Infrared spectroscopy analysis shows that the hydroxybenzenedisulfonamide derivatives of chitosan sulfate (see Figure 4 ) and chitosan sulfate (see figure 1 ) compared to 1398.06cm -1 The new absorption peak, which is the characteristic absorption peak of the hydroxybenzenedisulfon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com