Method for preparing gram-negative pathogenic bacteria inactivated vaccine having cross-protection power
A Gram-negative, inactivated vaccine technology, which is applied in the field of preparation of Gram-negative pathogenic bacteria inactivated vaccines, can solve the problems of high vaccine cost, inability to be widely used, and complicated outer membrane protein extraction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
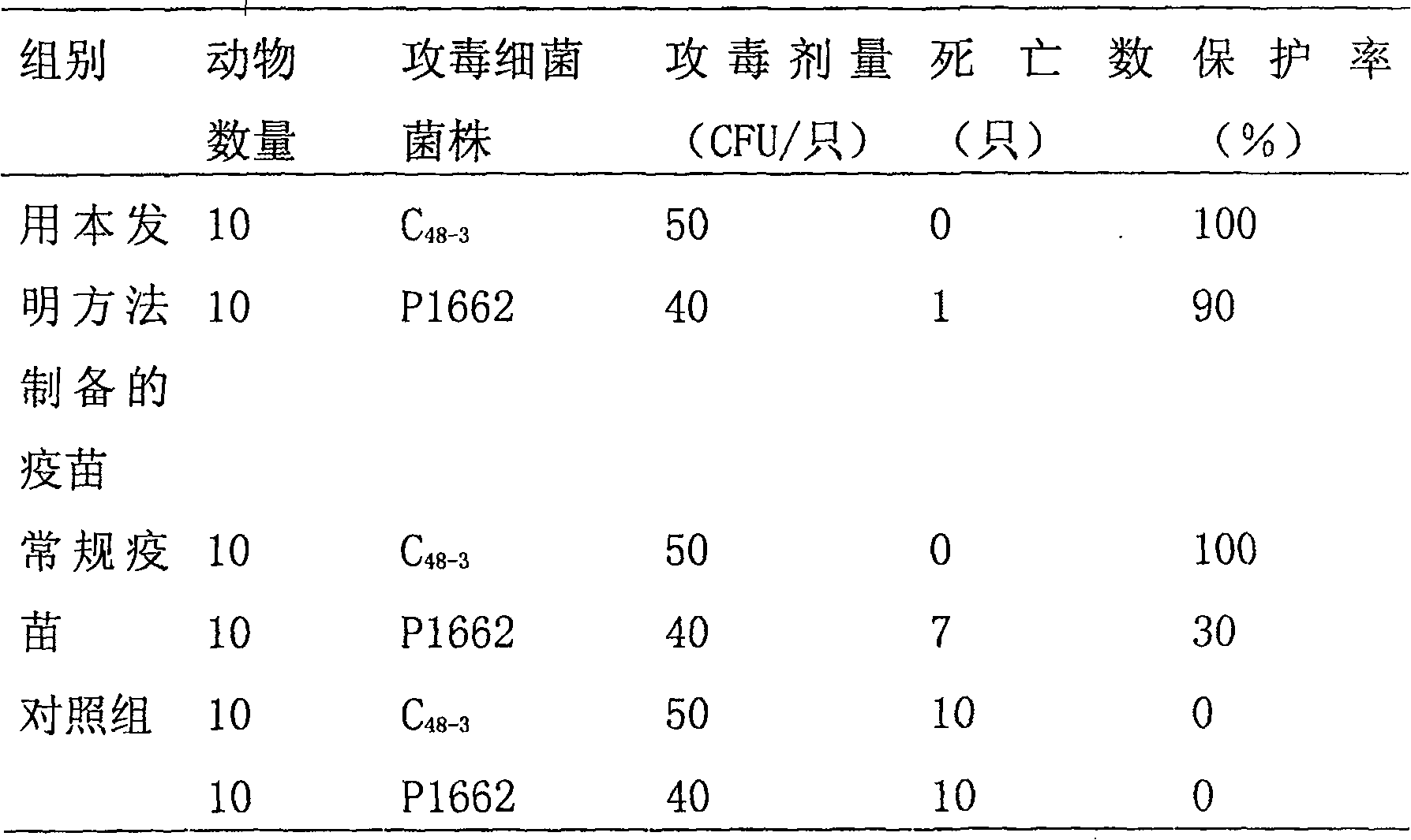
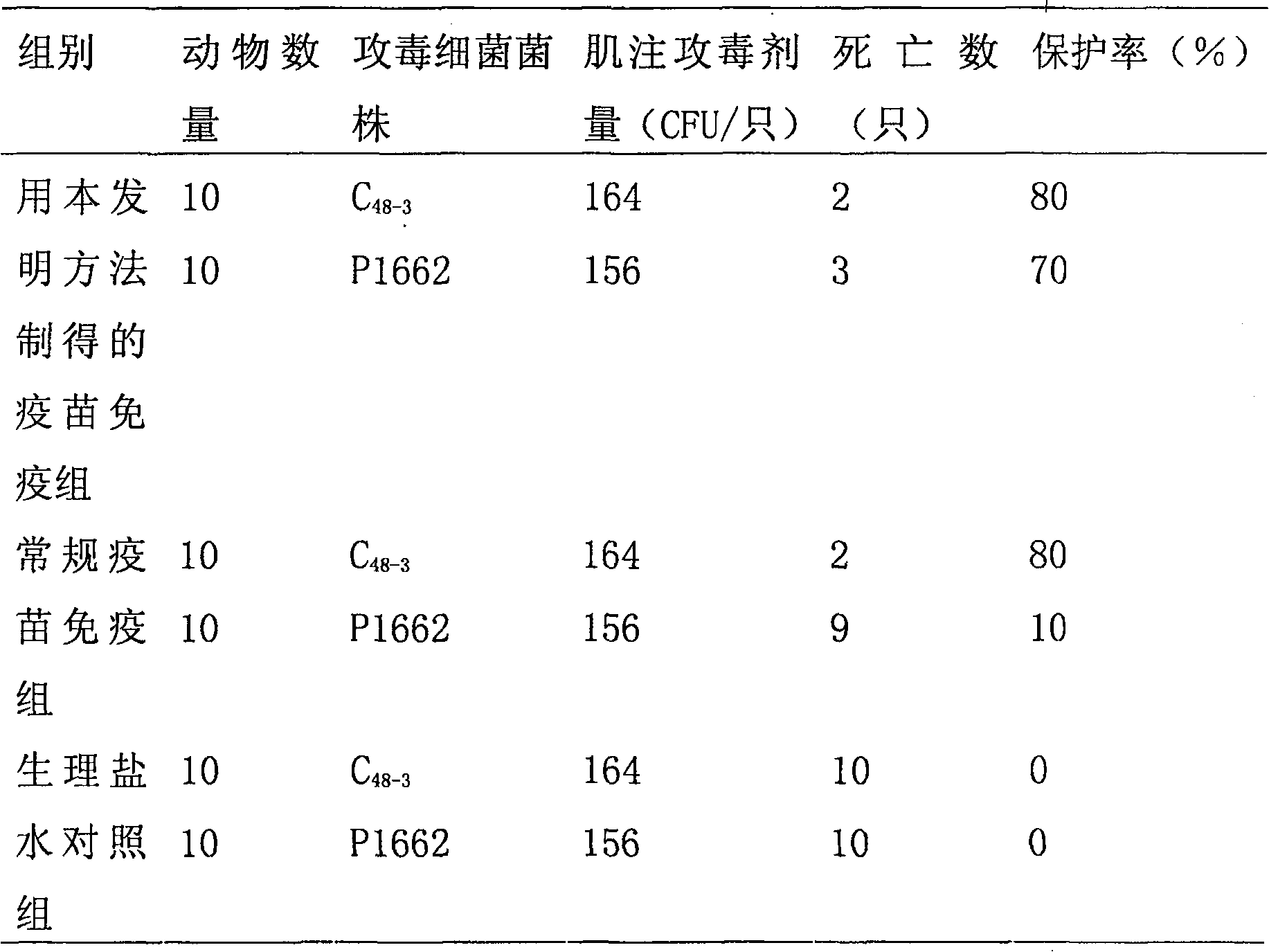
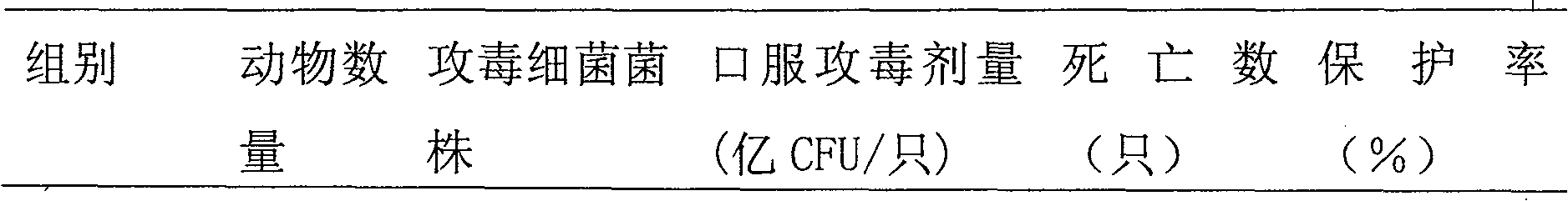
[0004] Embodiment 1: The present invention is used in the preparation method of fowl cholera propolis inactivated vaccine and its immunoprotective experiment. The virulent freeze-dried strain of Pasteurella multocida type A involved in this example was purchased from the China Veterinary Drug Control Institute, and was identified and preserved by the institute. 1. Preparation of bacteria solution for strain rejuvenation and seedling preparation:
[0005] After the freeze-dried bacteria were dissolved in 1ml of sterile physiological saline, they were streaked and inoculated on the blood Martin agar medium, and cultured at 37°C for 20-22 hours. The grown bacteria were washed and rejuvenated by continuous passage twice in the chicken body. The liver disease material was taken and inoculated on the blood Martin agar plate by streaking, and cultured at 37°C for 20-22 hours. 4-6 typical colonies were streaked and inoculated on blood Martin agar slant, and cultured at 37°C for 20-22...
Embodiment 2
[0023] Embodiment two: the present invention is used for the preparation method of Escherichia coli inactivated vaccine and its immunoprotective experiment
[0024] The standard Escherichia coli CMCC44115 strain freeze-dried bacterial classification that this embodiment relates to (serotype is O 2 type), purchased from the China Veterinary Drug Control Institute, identified and preserved by the institute.
[0025] 1. Rejuvenation of strains and preparation of bacterial solution for seedling production
[0026]After the freeze-dried bacteria were dissolved in 1ml of sterile physiological saline, they were streaked and inoculated on the blood Martin agar medium, and cultured at 37°C for 20-22 hours. The grown bacteria were washed and rejuvenated by continuous passage twice in the chicken body. The liver disease material was taken and inoculated on the blood martin agar culture plate by streaking, and cultured at 37°C for 20-22 hours. 4-6 typical colonies were streaked and inoc...
Embodiment 3
[0037] Embodiment 3: The present invention is used in the preparation method of the R. anatipestifer vaccine and its immune protection experiment.
[0038] The freeze-dried strain of R. anatipestifer (serotype I) involved in this example was isolated from clinically dead ducks.
[0039] 1. Rejuvenation of strains and preparation of bacterial solution for seedling production
[0040] Freeze-dried strains were dissolved in 1ml sterile saline, inoculated in serum nutrient broth, and cultured at 37°C for 16-18 hours. Take the culture solution and inoculate it on a serum nutrient agar plate, and incubate at 37°C for 16-18 hours. The grown bacteria were washed with sterilized saline and used as seed solution. The seed liquid is inoculated into the broth culture medium containing heme serum used for making the vaccine according to the ratio of 5% of the culture fluid volume, and 200 μmol / L 2,2'bipyridyl has been added in the broth culture medium as an iron chelating agent. It can ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com