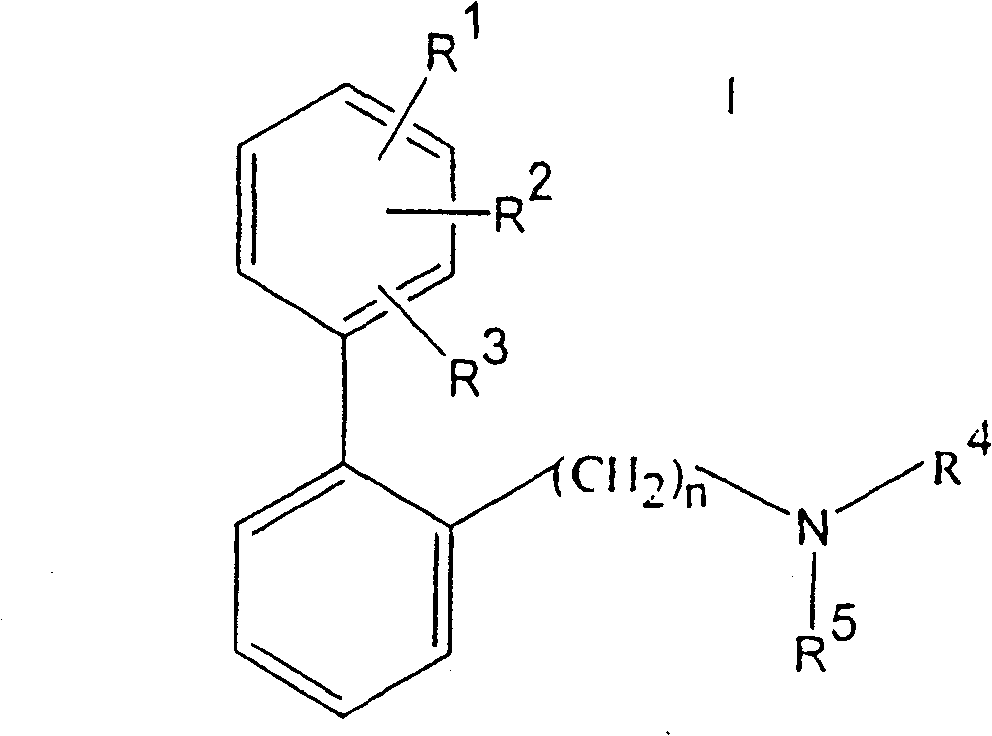
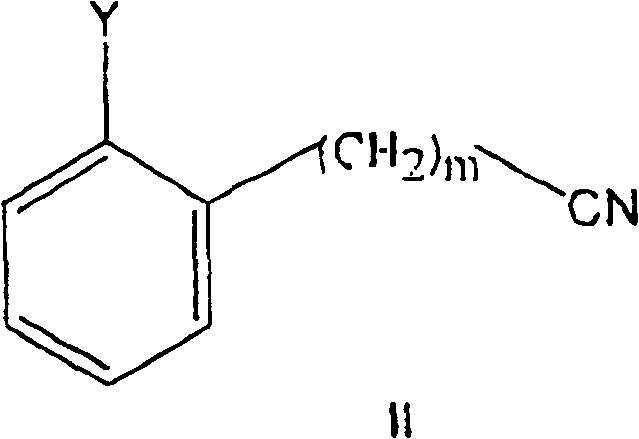
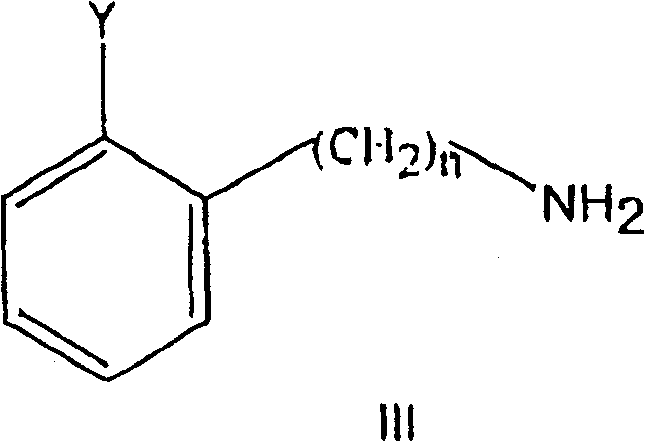
Substituted 2-dialkylaminoalkylbiphenyl derivatives
A technology of alkylaminoalkylbiphenyl and derivatives, which is applied in the field of preparation of medicines and can solve problems such as drug resistance, dependence, and addiction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] (3′-Methoxybiphenyl-2-ylmethyl)dimethylamine hydrochloride
[0106] first step
[0107] 3-Methoxyphenylboronic acid
[0108]41.3g (220mmol) of 3-bromoanisole was dissolved in 880ml of tetrahydrofuran, and the resulting solution was cooled to -70°C in a cold bath (ethanol / dry ice). 160ml (250mmol) of butyl lithium solution (1.6M in hexane) was added dropwise under nitrogen and the temperature was not higher than -60°C. After stirring at -70°C for 1.5 hours, 75 ml (660 mmol) of trimethyl borate was added dropwise and the temperature was not higher than -60°C. After stirring was continued for one hour in the cold bath, the temperature of the mixture was raised to 25°C over a period of two hours, 720 ml (1M) of hydrochloric acid were added and the resulting mixture was stirred at 25°C for 15 hours. For further completion of the reaction, the mixture was extracted three times with 300ml ether each time, the organic phases were combined, washed with 100ml each of water and...
Embodiment 2
[0116] (4'-Chlorobiphenyl-2-ylmethyl)dimethylamine hydrochloride
[0117] 0.88g (5.65mmol) 4-chlorophenylboronic acid, 1.27g (5.93mmol) (2-bromobenzyl) dimethylamine and 2.00g (18.8mmol) sodium carbonate obtained according to the method of embodiment 1 (the 2nd step) Dissolved in a mixture of 39ml toluene, 16ml water and 8ml ethanol. Under a nitrogen atmosphere, 133 mg of tetrakis(triphenylphosphine)palladium(0) was added, and the mixture was heated under reflux (bath temperature: 110° C.) for 16 hours. To further complete the reaction, 65 ml of ether were added and the mixture was extracted three times with 65 ml of potassium hydroxide solution (0.5M). The combined aqueous solution was re-extracted with 20 ml of ether, the resulting organic phases were combined, dried over anhydrous magnesium sulfate, then filtered, and the filtrate was concentrated using a rotary evaporator (500-10 mbar). This gave 1.30 g of crude base (93.8% of theory), which was transferred to a 3 x 25 c...
Embodiment 3
[0119] 2′-Dimethylaminomethylbiphenyl-3-ol hydrochloride
[0120] 0.70 (2.52 mmol) (3'-methoxybiphenyl-2-ylmethyl) dimethylamine hydrochloride prepared according to the method of Example 1 (the third step) was dissolved in 10 ml of water, and was oxidized with 10 ml of water and 2 ml of hydroxide Sodium solution (32 wt.%) liberates the base, the mixture is extracted three times with 20 ml of ether each time, the combined organic phases are dried over anhydrous magnesium sulfate, then filtered, and the filtrate is concentrated on a rotary evaporator (500-10 mbar). 0.59 g (2.44 mmol) of this base and 55 ml of hydrobromic acid solution (48 wt % aqueous solution) were heated under reflux (bath temperature 145° C.) for two hours. For further completing the reaction, the mixture is poured into 600ml sodium bicarbonate solution (1M) (pH7-8), the mixture is extracted three times with 100ml ethyl acetate each time, the organic phases are combined, dried with anhydrous magnesium sulfate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com