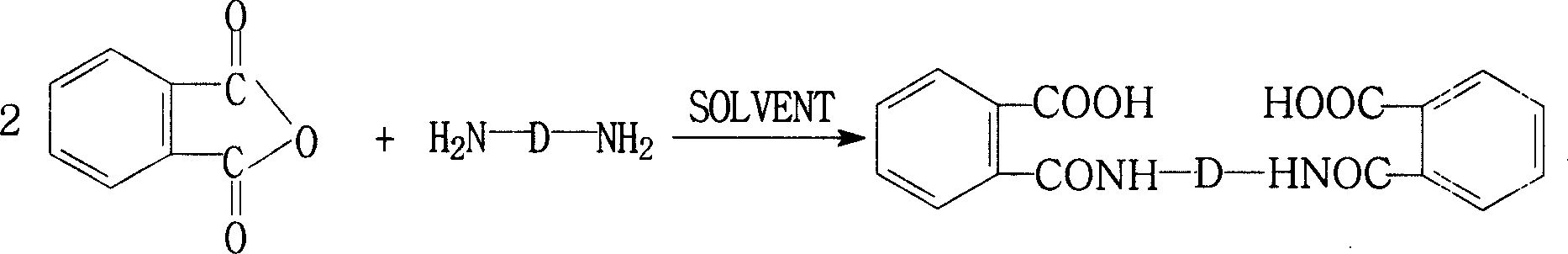
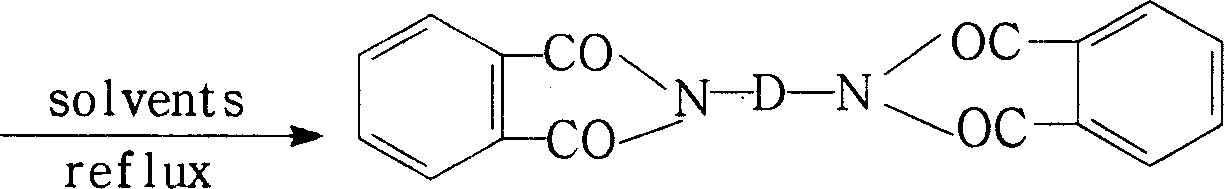
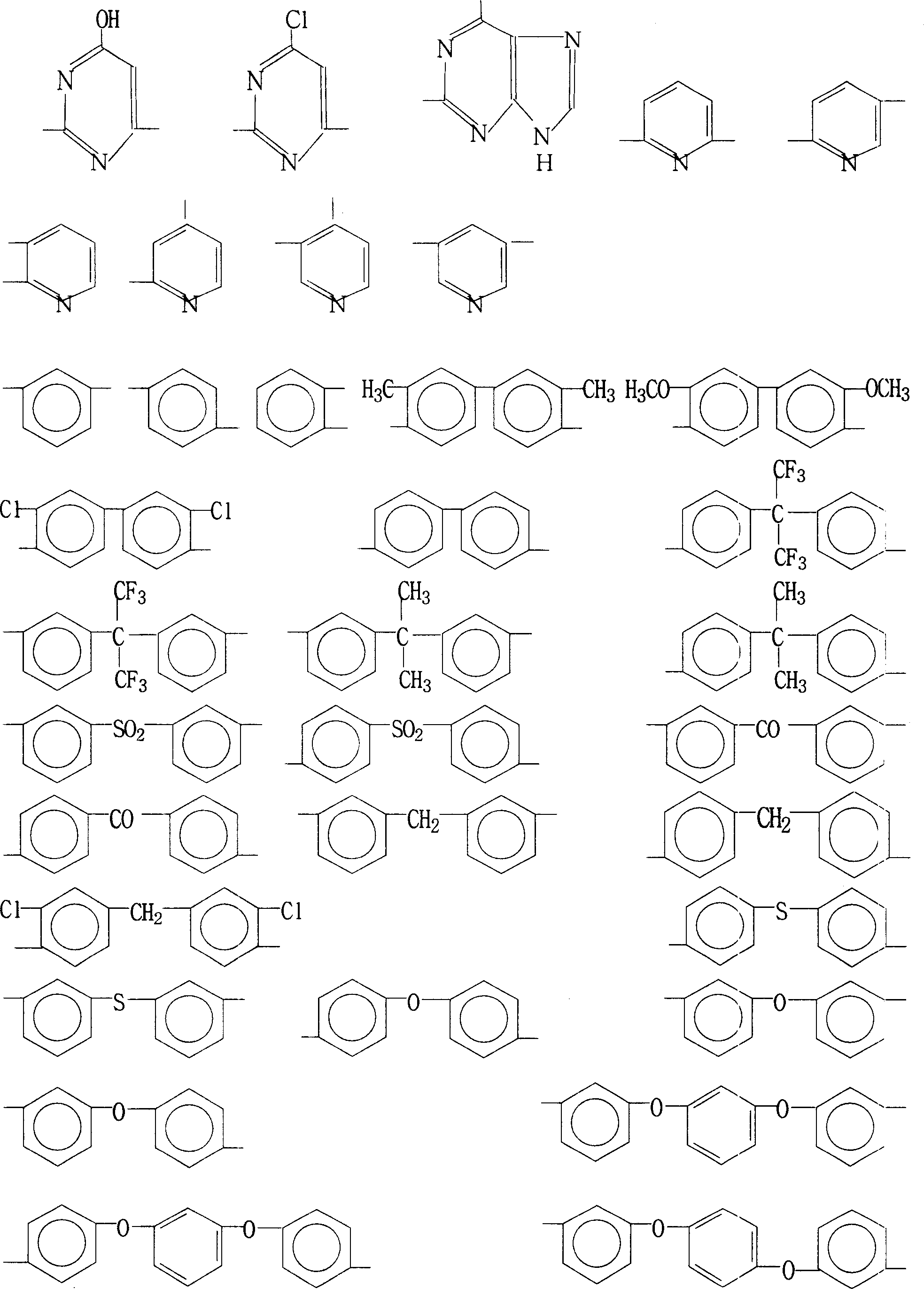Process for preparing biphthalimide
A bisphthalimide and primary amine technology, which is applied in the field of preparation of organic compounds, can solve problems such as no published literature or patent reports, and achieve simple operation, no environmental pollution, high product yield and purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 60 grams (1.0 moles) of ethylenediamine (EDA) and 2000 milliliters of N-methyl-2-pyrrolidone (NMP) were added together in the reaction kettle, after stirring at room temperature, the ice-water bath was cooled to keep the temperature in the reaction system at Within the temperature range of 0°C to 10°C, add phthalic anhydride (PA) in batches, totaling 370 g (2.5 moles), stir and react at 0°C to 10°C for 5 hours, add 800 ml of xylene, and stir , heated up to 140°C, refluxed water separation reaction for 10 hours, turned off the heating system, stirred, naturally cooled to room temperature, and precipitated a white solid product, filtered, rinsed twice with an appropriate amount of cold NMP solvent, and dried to obtain 313.6 grams ( Yield: 98%) white crystals of N,N'-(1,2-ethylene)bisphthalimide with a purity of 99.8%.
Embodiment 2
[0034]74 grams (1.0 moles) of 1,3-propanediamine (13PDA) and 2000 milliliters of N,N-dimethylacetamide (DMAc) were added to the reaction kettle together, after stirring at room temperature, the ice-water bath was cooled to make the reaction The temperature in the system was maintained within the temperature range of 0°C to 10°C, and phthalic anhydride (PA) was added in batches, totaling 325.6 grams (2.2 moles). After stirring and reacting at 0°C to 10°C for 3 hours, 2000 Milliliter of toluene, stirred, heated up to 120 ° C, refluxed water separation reaction for 8 hours, turned off the heating system, stirred, naturally cooled to room temperature, a white solid product was precipitated, filtered, rinsed twice with an appropriate amount of cold DMAc solvent, and dried. 317.3 g (95% yield) of white N,N'-(1,3-propylene)bisphthalimide crystals were obtained with a purity of 99.6%.
Embodiment 3
[0036] Add 23.8 g (0.1 mol) of 3,3'-dimethyl-4,4'-diaminodicyclohexylmethane (DMDACM) and 300 ml of N,N-dimethylformamide (DMF) into the reaction kettle together , after stirring evenly at room temperature, cooling in an ice-water bath to maintain the temperature in the reaction system within the temperature range of 0°C to 10°C, adding phthalic anhydride (PA) in batches, a total of 32.6 grams (0.22 moles), at 0 After stirring and reacting at ℃~10℃ for 1 hour, add 600 ml of toluene, stir, heat up to 110℃, reflux and water separation reaction for 10 hours, turn off the heating system, stir, and naturally cool to room temperature, a white solid product is precipitated, filtered, Rinse twice with an appropriate amount of cold DMF solvent, and dry to obtain 47.8 grams (96% yield) of white N, N'-[4-methylene-bis(3-methylcyclohexyl)]bisphthaloimide Amine crystals with a purity of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com