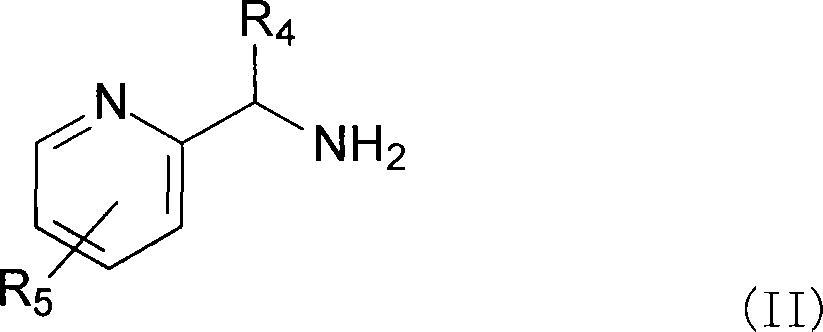
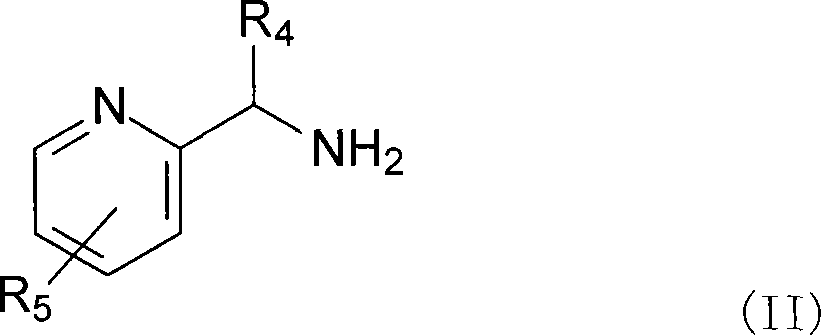
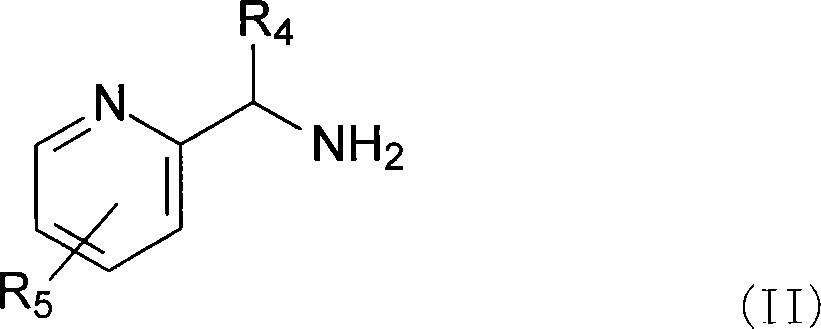
Complexes of ruthenium with 2-(aminomethyl) pyridines and phosphines, their preparation and use as catalysts
A complex and aminomethyl technology, applied in the field of ruthenium complexes, can solve the problem of low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0083] Example 1: trans-RuCl 2 (PPh 3 ) 2 [2-(H 2 NCH 2 )C 5 h 4 Synthesis of N](3)
[0084] The complex RuCl 2 (PPh 3 ) 3 (1) (0.400g, 0.417mmol) was suspended in 5ml of distilled dichloromethane, and reacted with 2-(aminomethyl)pyridine (45L, 0.436mmol). After the reaction mixture was stirred at room temperature for 2 hours, the volume of the solution was reduced by half, and then 5 ml of pentane was added to precipitate the complex. The resulting solid was filtered off, washed twice with 10 ml of ether, and dried under reduced pressure.
[0085] Yield 250 mg (75%). C 42 h 38 Cl 2 N 2 P 2 Elemental analysis of Ru., calculated as (%): C, 62.69; H, 4.76; N, 3.48; found C, 62.85; H, 4.80; N, 3.54. 1 H nuclear magnetic resonance (NMR) analysis (200.1MHz, CDCl 3 , 20°C, TMS): δ8.53(d, J(HH)=4.2Hz, 1H; o-C 5 h 4 N), 7.60-6.50 (m, 33H; aromatic proton), 4.46 (broad s, 2H; CH 2 ), 3.29 (width s, 2H; NH 2 ). 13 C{ 1 H} NMR analysis (50.3MHz, CDCl 3 , 20°C, TMS...
example 2
[0086] Example 2: Complex trans-RuHCl (PPh 3 ) 2 [2-(H 2 NCH 2 )C 5 h 4 Synthesis of N](4)
[0087] The complex RuHCl (PPh 3 ) 3 (211mg, 0.228mmol) was suspended in 10ml of n-heptane, reacted with 2-(aminomethyl)pyridine (24L, 0.233mmol), and then heated under reflux for 1 hour. The yellow product was filtered off, washed with n-heptane (3 x 5 ml) and dried under reduced pressure.
[0088] Yield: 118mg (67%).C 42 h 39 ClN 2 P 2 Elemental analysis (%) of Ru, calculated as: C, 65.49; H, 5.10; N, 3.64; found: C, 65.23; H, 5.03; N, 3.41. 1 H NMR analysis (200.1MHz, CD 2 Cl 2 , 20°C, TMS): δ8.20(s, 1H; o-C 5 h 4 N), 7.70-6.40 (m, 33H; aromatic proton), 4.30 (false t, J(HH)=14.1Hz, 1H; CH 2 ), 4.07(d, J(HH)=14.3Hz, 1H; CH 2 ), 2.87 (false t, J(HH)=10Hz, 1H; NH 2 ), 2.20 (false d, J(HH)=10Hz, 1H; NH 2 ), -17.70 (dd, J(HP)=23.5, 29.7Hz). 13 C{ 1 H} NMR analysis (50.3MHz, CD 2 Cl 2 , 20°C, TMS): δ159.7(s; NCCH 2 ), 155.6(d, J(CP)=4.0Hz; NCH), 138.8-118.7(m; aro...
example 3
[0089] Example 3: Complex cis-RuCl 2 (PPh 3 ) 2 [2-(H 2 NCH 2 )C 5 h 4 Synthesis of N](5)
[0090] The complex RuCl 2(PPh 3 ) 3 (1) (1.34g, 1.40mmol) was suspended in 10ml of toluene, and reacted with 2-(aminomethyl)pyridine (0.160ml, 1.55mmol). The reaction mixture was heated under reflux for 2 hours, the volume of the solution was reduced by half, and the complex was precipitated after adding 5 ml of pentane. The filtered solid product was washed twice with 5 ml of ether and dried under reduced pressure.
[0091] Yield: 750 mg (66.4%).C 42 h 38 Cl 2 N 2 P 2 Elemental analysis (%) of Ru, calculated as: C, 62.69; H, 4.76; N, 3.48; found: C, 62.31; H, 4.87; N, 3.60. 1 H NMR analysis (200.1MHz, CD 2 Cl 2 , 20°C, TMS): δ9.16(d, J(HH)=5.7Hz, 1H; positive-C 5 h 4 N), 7.70-6.89 (m, 33H; aromatic proton), 3.65 (m, 2H; CHHNHH), 3.00 (m, 1H, CH 2 ), 1.42 (m, 1H, NH 2 ). 31 P{ 1 H} NMR analysis (81.0MHz, CD 2 Cl 2 , 20°C, H 3 PO 4 ): δ 50.5 (d, J(PP)=33.4Hz), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com