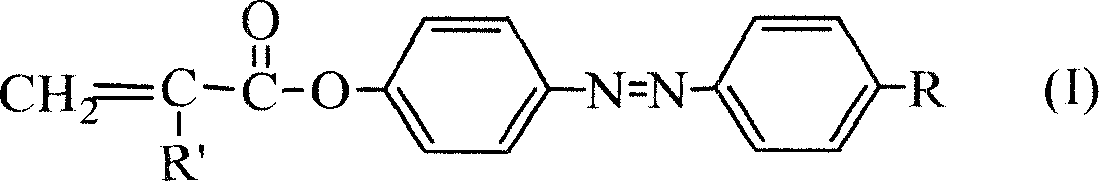
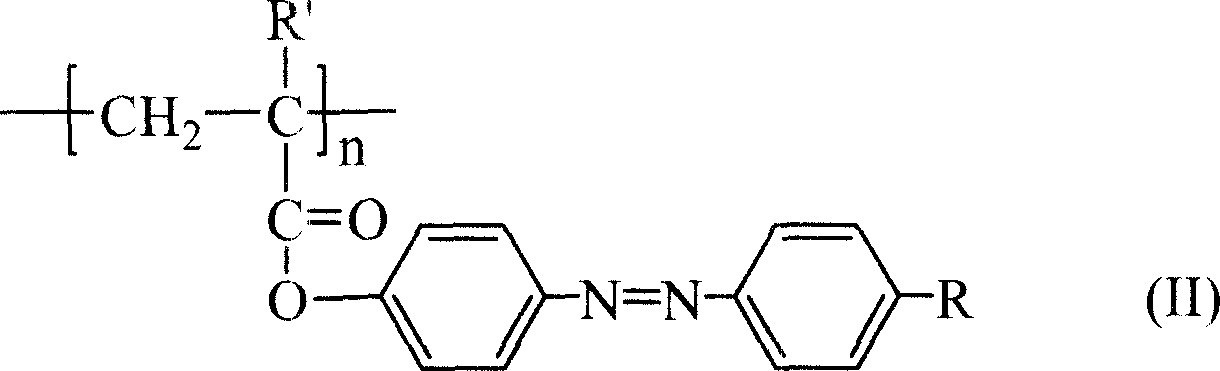
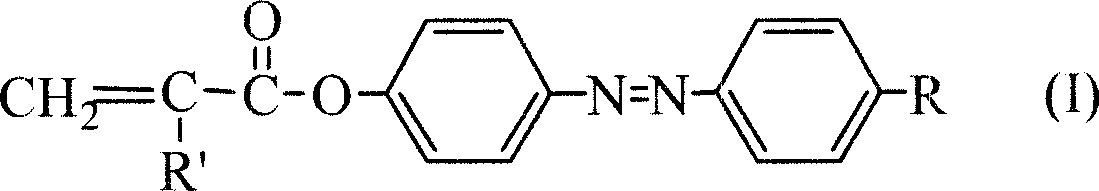
Azo monomer and polymer with third order non-linear optical property
A technology of third-order nonlinearity and optical properties, which is applied in the field of polymer preparation by atom transfer radical polymerization, can solve the problems of low content of functional groups and influence on polymer performance, and achieve controllable molecular weight and narrow molecular weight distribution , good film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take 2-3g of p-nitroaniline to prepare hydrochloride solution, cool in an ice bath, add dropwise 10g of 15-20% sodium nitrite aqueous solution, and react under ice bath for half an hour to obtain p-nitroaniline diazonium salt solution. Take 2 g of phenol and 2 g of sodium hydroxide, add 100 g of water and stir to dissolve, cool in an ice bath, add the above diazonium salt solution dropwise, and react in an ice bath for 4 hours. Filter and wash with plenty of water to obtain p-nitroazo intermediate. The third-order nonlinear susceptibility coefficient of a sample of this intermediate measured by four-wave mixing method is 4.87×10 -12 esu.
[0033] Take 2-3g of p-nitroazo intermediate, dissolve it in 40ml of tetrahydrofuran, add 2ml of triethylamine, and stir evenly under ice bath. Take 1-2 g of acryloyl chloride and slowly add it dropwise to the above solution, react in ice bath for 1 hour, then continue to stir at room temperature for 4 hours. The reaction solution w...
Embodiment 2
[0037] Take 2-3g of p-methoxyaniline to make hydrochloride solution, put it in ice bath to cool, add dropwise 10g of 15-20% sodium nitrite aqueous solution, and react under ice bath for half an hour to obtain p-methoxyaniline diazonium salt solution. Take 2 g of phenol and 2 g of sodium hydroxide, add 80-100 g of water and stir to dissolve, cool in an ice bath, add the above diazonium salt solution dropwise, and react in an ice bath for 4 hours. Filtration and washing with a large amount of deionized water gave p-methoxyazo intermediate. The third-order nonlinear susceptibility coefficient of a sample of this intermediate measured by four-wave mixing method is 4.16×10 -12 esu.
[0038] Take 2-3g of p-methoxyazo intermediate, dissolve it in 20-30ml tetrahydrofuran, add 2-3ml triethylamine, and stir evenly under ice bath. Take 1-2 g of acryloyl chloride and slowly add it dropwise to the above solution, react in ice bath for 1 hour, then continue to stir at room temperature for ...
Embodiment 3
[0042] Take 3-4g of p-bromoaniline to prepare hydrochloride solution, cool in an ice bath, add dropwise 10g of 15-20% sodium nitrite aqueous solution, and react under ice bath for half an hour to obtain p-bromoaniline diazonium salt solution. Take 2 g of phenol and 2 g of sodium hydroxide, add 80-100 g of water to dissolve, cool in an ice bath, add the above diazonium salt solution dropwise, and react in an ice bath for 4 hours. Filtration and washing with a large amount of deionized water gave p-bromoazo intermediate. The third-order nonlinear susceptibility coefficient of a sample of this intermediate measured by four-wave mixing method is 4.36×10 -12 esu.
[0043] Take 3-4g of p-bromoazo intermediate, dissolve it in 30-40ml tetrahydrofuran, add 2-3ml triethylamine, and stir well under ice bath. Take 1-2 g of acryloyl chloride and slowly add it dropwise to the above solution, react in ice bath for 1 hour, then stir at room temperature for 4 hours. The reaction solution wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com