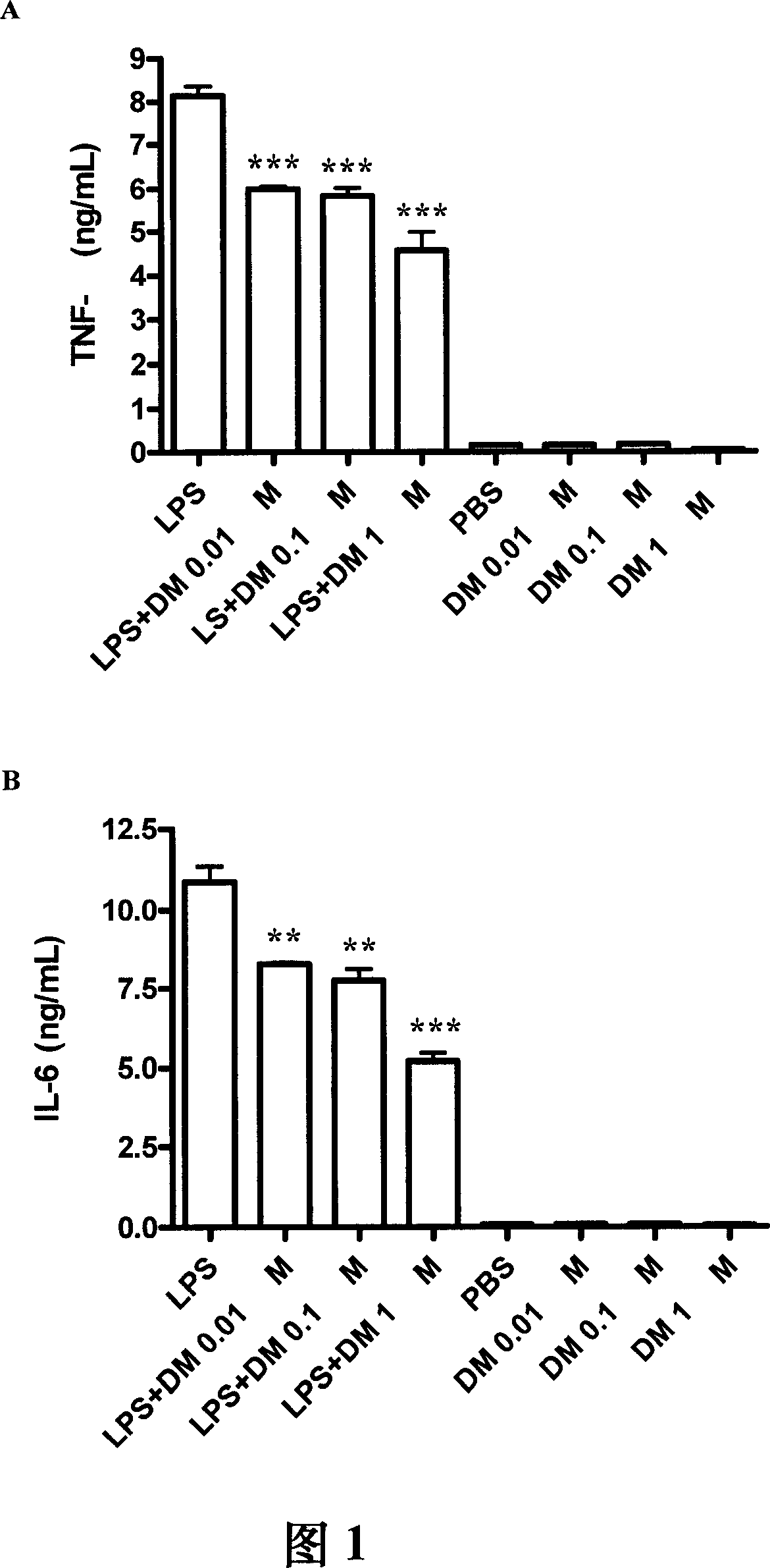
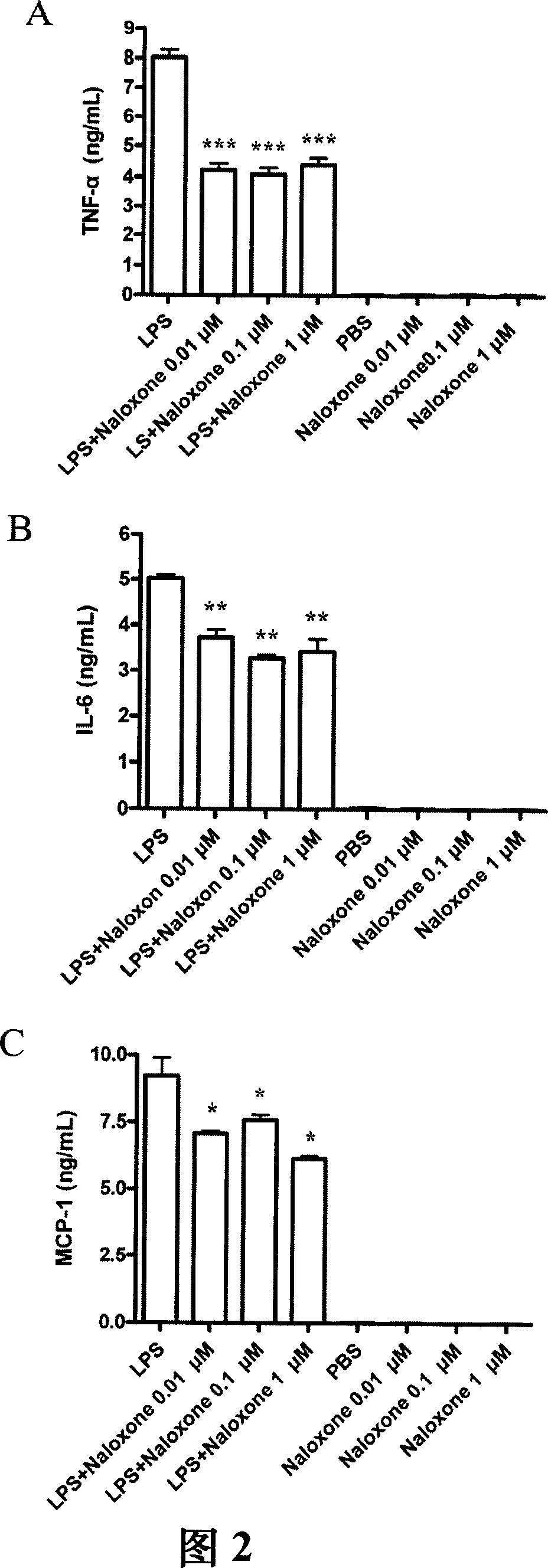
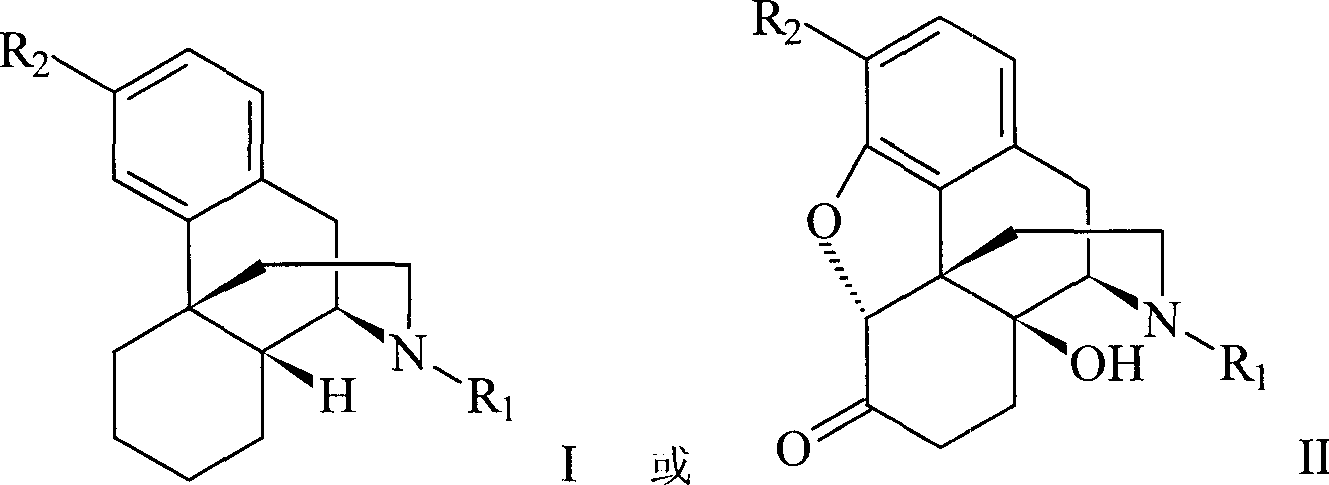
Methods and compositions for bactericide, bacteriostatic and anti-inflammation
A composition and compound technology, applied in the field of sterilization or bacteriostasis, can solve problems such as side effects that are not suitable for use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the antibacterial action of DM to Propionibacterium acnes (Gram-positive anaerobic bacteria)
[0060] A DM stock solution was prepared at 500,000 ppm (50%). Add 0.25g DM to 250μl DMSO, stir until fully mixed. 500,000 ppm DM was then serially diluted.
[0061] A 5 ml culture of P. acnes grown in RCM (Enhanced Clostridium Medium) was centrifuged. Dilute 20 times with culture medium and adjust the concentration of bacterial cells to approximately OD 595 =0.1×10 6CFU / ml. Take an equal amount of 200 μl culture medium containing bacterial cells and add to each well of the 96-well plate. The culture medium containing bacterial cells was mixed with 2 μl DM, and each concentration was sampled in triplicate. Bacteria were cultured at 37°C for 96 hours under anaerobic conditions, and then the OD was measured 595 . MIC refers to the lowest concentration of an antimicrobial agent sufficient to inhibit bacterial growth (eg, growth inhibition).
[0062] 0.1ml ...
Embodiment 2
[0069] Embodiment 2: DM is to the antibacterial action of Bacteroides fragilis (gram-negative anaerobic bacteria)
[0070] Bacteroides fragilis was grown anaerobically in thiosulfate broth for 18-24 hours and used to prepare a Mac Faland 0.5 bacterial cell suspension. The 0.5 g / ml DMSO stock solution was diluted with anaerobic thiosulfate broth. Add 1000 μl of anaerobic thiosulfate broth to 1000 μl of DM, dilute it at a ratio of 1:1, and then place it in an oxygen-free incubator for 24 hours.
[0071] Table 3: MIC test of Bacteroides fragilis (minimum inhibitory concentration test, the DM unit of the test is expressed in ppm and percentage%)
[0072] (+: growth inhibition; -: no growth inhibition)
[0073] DM (ppm)
[0074] "Negative control" means that no DM was added to the thiosulfate broth containing the bacteria.
Embodiment 3
[0075] Embodiment 3: the antibacterial action of DM to Gram-positive aerobic bacteria Escherichia coli
[0076] The matrix used for the antimicrobial disc diffusion susceptibility test was made of Mueller Hinton II agar (Becton, Dickinson and Company, France).
[0077] Prepare Mueller Hinton II agar by suspending 38 g of Mueller Hinton II agar powder in 1 liter of double distilled water. The above agar was autoclaved at 121°C for 15 minutes. Then pour into each agar plate and wait to set. 100 μl containing 1 x 10 5 The culture medium of CFU Escherichia coli was spread on the agar plate and cultivated for 96 hours.
[0078] Table 4: Growth Inhibition of Gram-positive Aerobic Bacteria by DM Disc Diffusion: Escherichia coli lawns were cultured at 37°C for 96 hours.
[0079] DM concentration
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com