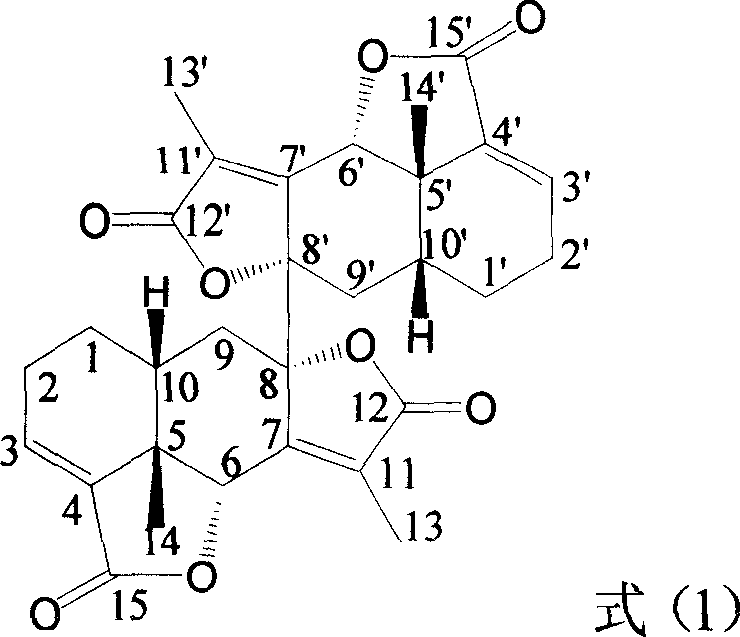
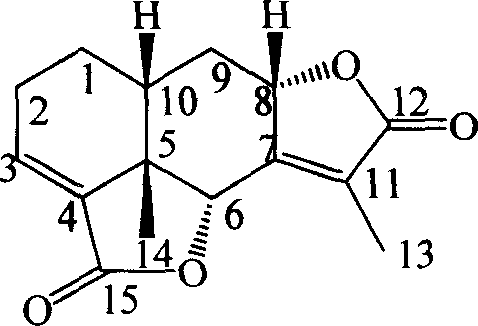
Eremophil-2-internal-ester diploymer having function of inhibiting tumor cell growth activity and use thereof
A technology of ester dimer and erimofantane, which is applied in the field of natural medicinal chemistry and pharmacology, can solve the problems that the biological activity research has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Compound of formula (1): 8α-[Erimofantane-3′, 7′(11′)-diene-12′, 8′α; 15′, 6′α-dilactone]-Erimophane Preparation of fenane 3,7(11)-diene-12,8α; 15,6α-dilactone
[0016] The underground part of the sample was sun-dried and pulverized (dry weight 5.0 kg), then heated twice under boiling with 95% industrial alcohol, the extracts were cooled and combined, and concentrated under reduced pressure to obtain 389 grams of tan viscous primary extract. Suspension was made with distilled water and sequentially distributed and extracted with petroleum ether, ethyl acetate and n-butanol. After the organic layers were evaporated to dryness under reduced pressure, 26 g, 127 g, and 89 g were obtained respectively, and the aqueous layer was 147 g.
[0017] Take 120 grams of ethyl acetate extraction extract, mix it with 150 grams of 200-300 mesh silica gel to evaporate the solvent, then go through silica gel column chromatography (2500 grams), and use chloroform-methanol syst...
Embodiment 2
[0029] Pharmacological embodiment 2: Cytotoxic activity of formula (1) compound on BEL-7404 cells
[0030] BEL-7404 (human liver cancer) cells were cultured with RPMI1640 medium containing 10% fetal bovine serum, 100 U / mL penicillin and 100 U / mL streptomycin. cells in 5×10 per well 3 concentration into a 96-well plate at 37 °C with 5% CO 2 Incubate for 24 hours in a humidified incubator.
[0031] Determination of cell viability by the modified MTT method, the specific method is as in Pharmacological Example 1. Wherein formula (1) compound is to BEL-7404 cell half inhibitory concentration (IC 50 ) obtained from the dose-response curve.
[0032] IC of the compound of formula (1) 50 For: 2.55×10 -4 M; while the IC of the positive control cisplatin on BEL-7404 cells 50 2.62×10 -5 M.
[0033] Experimental conclusion: This experiment shows that the arimorphine dilactone dimer has strong cytotoxicity to BEL-7404 cells, and it may be developed into a new drug with anti-live...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com