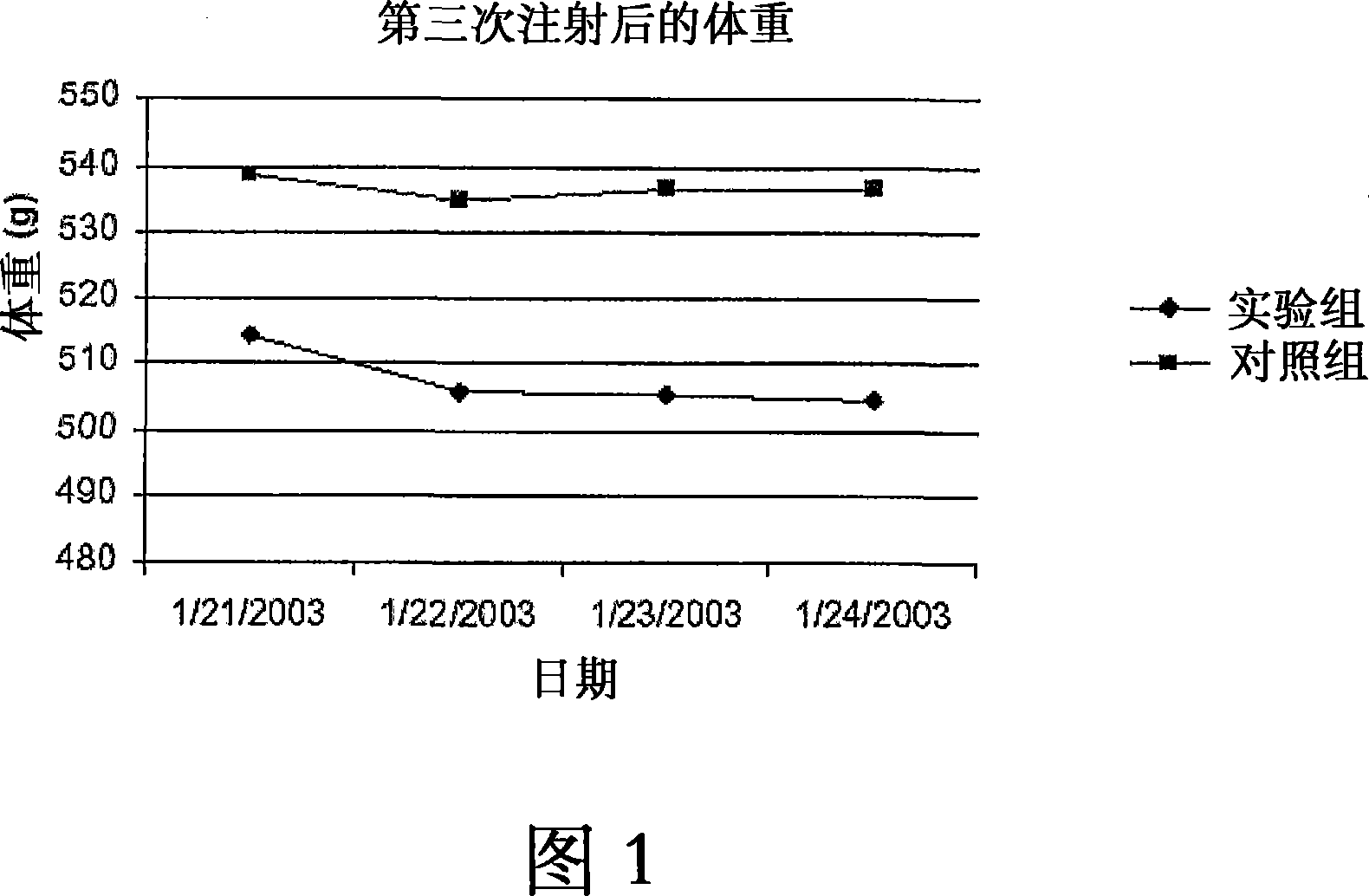
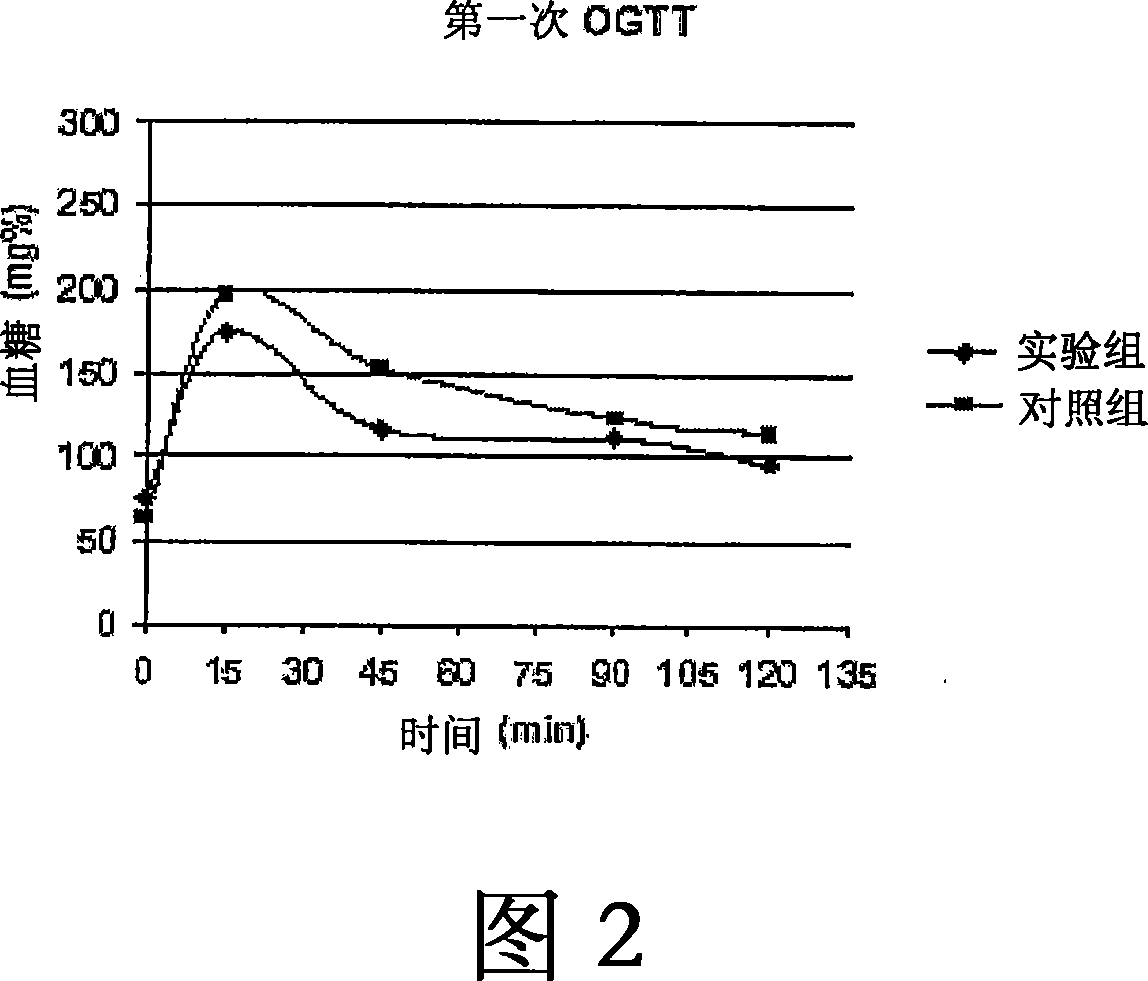
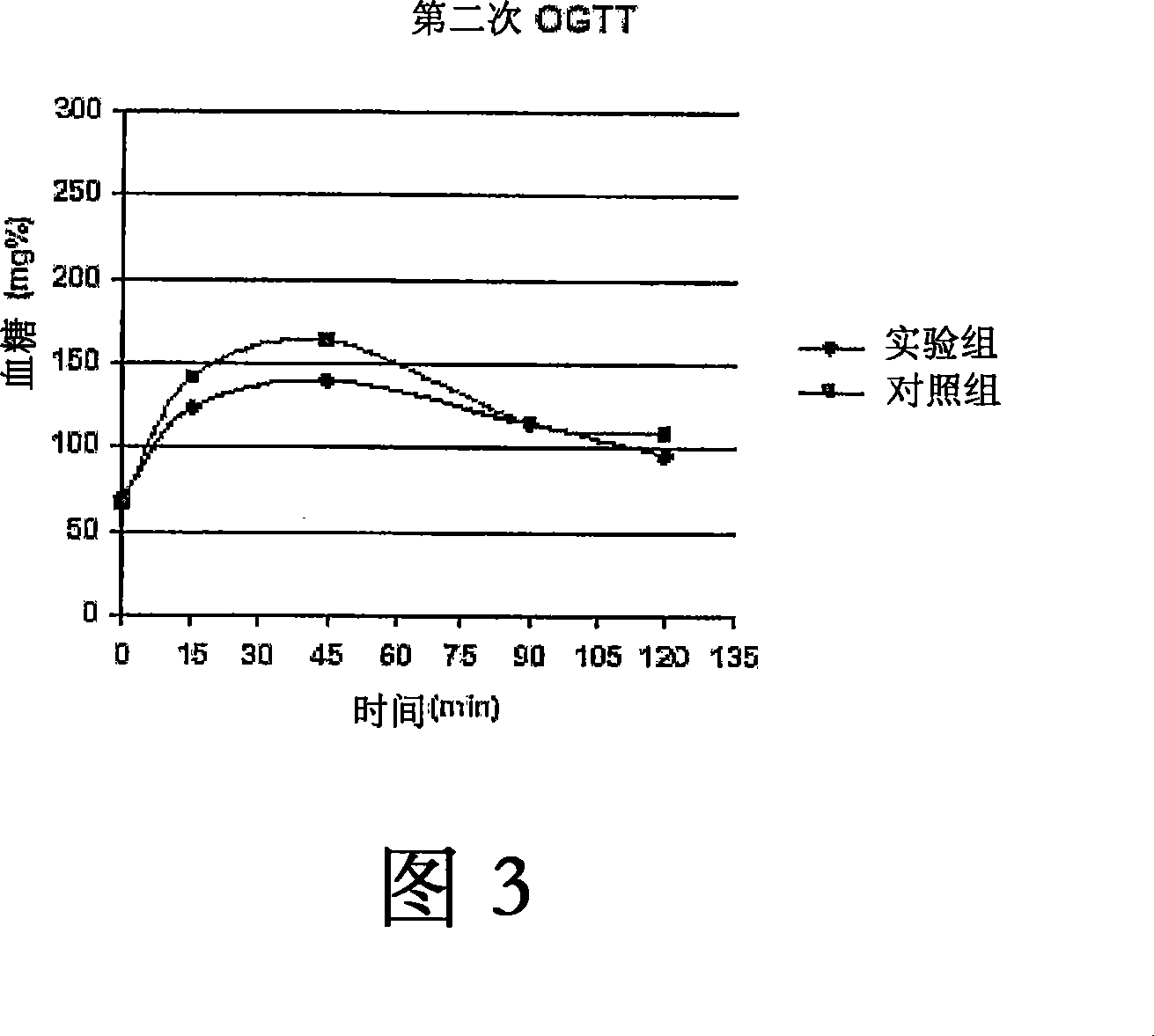
Use of targeted oxidative therapeutic formulation in treatment of diabetes and obesity
An oxidizing agent and diabetes technology, applied in the treatment of diabetes and obesity, in the field of pharmaceutical compositions or preparations, can solve the problems that geraniol peroxide does not show clinical efficacy, danger, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Ozonolysis of Unsaturated Hydrocarbons
[0074] The ozonolysis of olefins is carried out in solvent or neat. In both cases, cooling of the reaction mixture is critical in avoiding explosive decomposition of the peroxidic product of the reaction.
[0075] The following general procedure is typical for the ozonolysis of liquid olefins.
[0076] A 1 liter flask equipped with a magnetic stirrer was charged with olefin (2 moles) and the apparatus was weighed. The flask was placed in a cooling bath (ice-water or ice-salt). When the contents have cooled below 5°C, agitation is started and dry oxygen containing ozone (typically 3% ozone) is passed through the mixture. It is convenient to vent the ozone-containing oxygen through the glass frit, but this is not necessary for a solution that is being stirred. Gas flow is periodically interrupted for reaction vial weighing or reaction mixture sampling, after which gas flow is resumed.
[0077] When the weight of the...
Embodiment 2
[0080] Embodiment 2: the preparation of pharmaceutical preparation
[0081] The preferred pharmaceutical formulation of the present invention is prepared as follows:
[0082] (1) Ozone / pure oxygen gas mixture 120mg / L is bubbled through dienol, 3,7-dimethyl-2,6-octadien-1-ol (geraniol), 1 liter / hour;
[0083] (2) The reaction temperature is maintained at about 5°C;
[0084] (3) A small portion of the reaction product is taken out every 1 hour and passed through H 1 NMR to detect the formation of peroxides or reaction products;
[0085] (4) When there are more than 50% available unsaturated bond reactions, stop the reaction;
[0086] (5) Dilute the product mixture (1:10) with dimethyl sulfoxide to form a solution or dispersion;
[0087] (6) Before being applied to the target biological system, add a sufficient amount of dry powder mixture of hematoporphyrin, rose bengal and menaquinone to the solution or dispersion, and spread it in the target biological system when injected...
Embodiment 3
[0088] Embodiment 3: the example of pharmaceutical preparation
[0089] Two preferred formulations are as follows:
[0090] a.
[0091] weight%
[0092] *Detected by mass spectrometry
[0093] b.
[0094] weight%
[0095] *Detected by mass spectrometry
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com