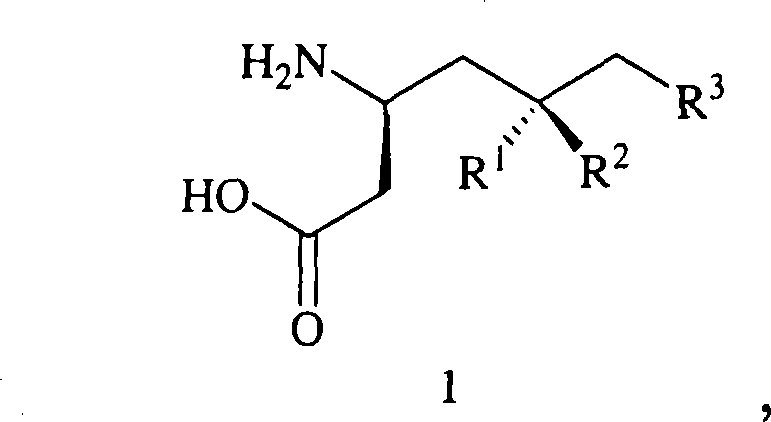
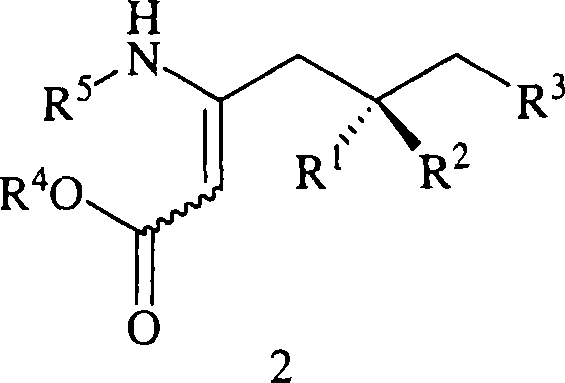
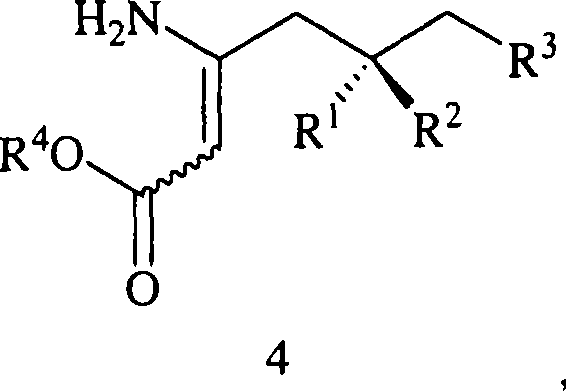
Preparation of beta-amino acids having affinity for the alpha-2-delta protein
A technology of alkyl and aryl groups, applied in the field of materials for preparing optically active β-amino acids, can solve problems such as preparation difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0305] Example 1. Preparation of (R)-3-methyl-hexanoic acid from (R)-2-methyl-pentan-1-ol via (R)-3-methyl-capronitrile
[0306] (R)-2-Methyl-pentan-1-ol, MeCl 2 The mixture with pyridine was cooled to 0°C to 10°C. To this mixture was added tosyl chloride and the resulting reaction mixture was allowed to warm to room temperature overnight. The reaction was quenched by adding water and the mixture was separated into upper and lower layers. The lower layer was washed with dilute aqueous HCl. The organic layer was concentrated to an oil by vacuum distillation, which was then diluted with DMSO and allowed to react by adding sodium cyanide and heating to 50 °C for 3 hours. After cooling to room temperature, the reaction mixture was diluted with hexane and water and the upper layer was washed with water. The hexane layer was concentrated by vacuum distillation to yield (R)-3-methyl-capronitrile as an oil. The oil was reacted by adding aqueous HCl and heating the mixture to 50-6...
Embodiment 2
[0307] Example 2. Preparation of (R)-5-methyl-3-oxo-octanoic acid ethyl ester from (R)-methyl-hexanoic acid and potassium ethylmalonate
[0308] To a stirred mixture of N,N carbonyldiimidazole (26.4 g) in THF (175 mL) was added (R)-3-methyl-hexanoic acid (20 g) at room temperature. The reaction mixture became clear after stirring at room temperature for 4 hours to give a solution of the active carboxylic acid. To a stirred mixture of potassium ethylmalonate (49.6 g) in acetonitrile (265 mL) was added Et 3 N (21.4 mL). To this mixture was added anhydrous magnesium chloride powder (35.1 g) in portions while maintaining the temperature at 15°C to 25°C. The resulting slurry was allowed to warm to room temperature and allowed to stir for 4 hours. The activated carboxylic acid solution was added to the slurry and the mixture was stirred at room temperature for 17 hours. The reaction was quenched with aqueous HCl and extracted with MTBE. With sodium bicarbonate and NaCl / H 2 The...
Embodiment 3
[0309] Example 3. Preparation of (R)-5-methyl-3-oxo-octanoic acid ethyl ester from (R)-methyl-hexanoic acid and potassium ethylmalonate
[0310] To a stirred mixture of N,N carbonyldiimidazole (33 kg) in EtOAc (217 L) was added (R)-3-methyl-hexanoic acid (25 kg) dissolved in EtOAc (30 L) at room temperature. The reaction mixture became clear after stirring at room temperature for 1 hour to give a solution of the active carboxylic acid. To a stirred mixture of potassium ethylmalonate (45.8 kg) and magnesium chloride (25.6 kg) in EtOAc (320 L) was added Et 3 N (31.1 kg). The activated carboxylic acid solution was added to this slurry and the resulting mixture was stirred at 40°C to 50°C for 11 hours. The mixture was cooled to 10°C to 15°C and quenched with aqueous HCl. The organic layer was washed sequentially with water and aqueous sodium bicarbonate and then concentrated by vacuum distillation to give the title compound as a yellow oil (38.2 kg, 92% pure product based on 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com