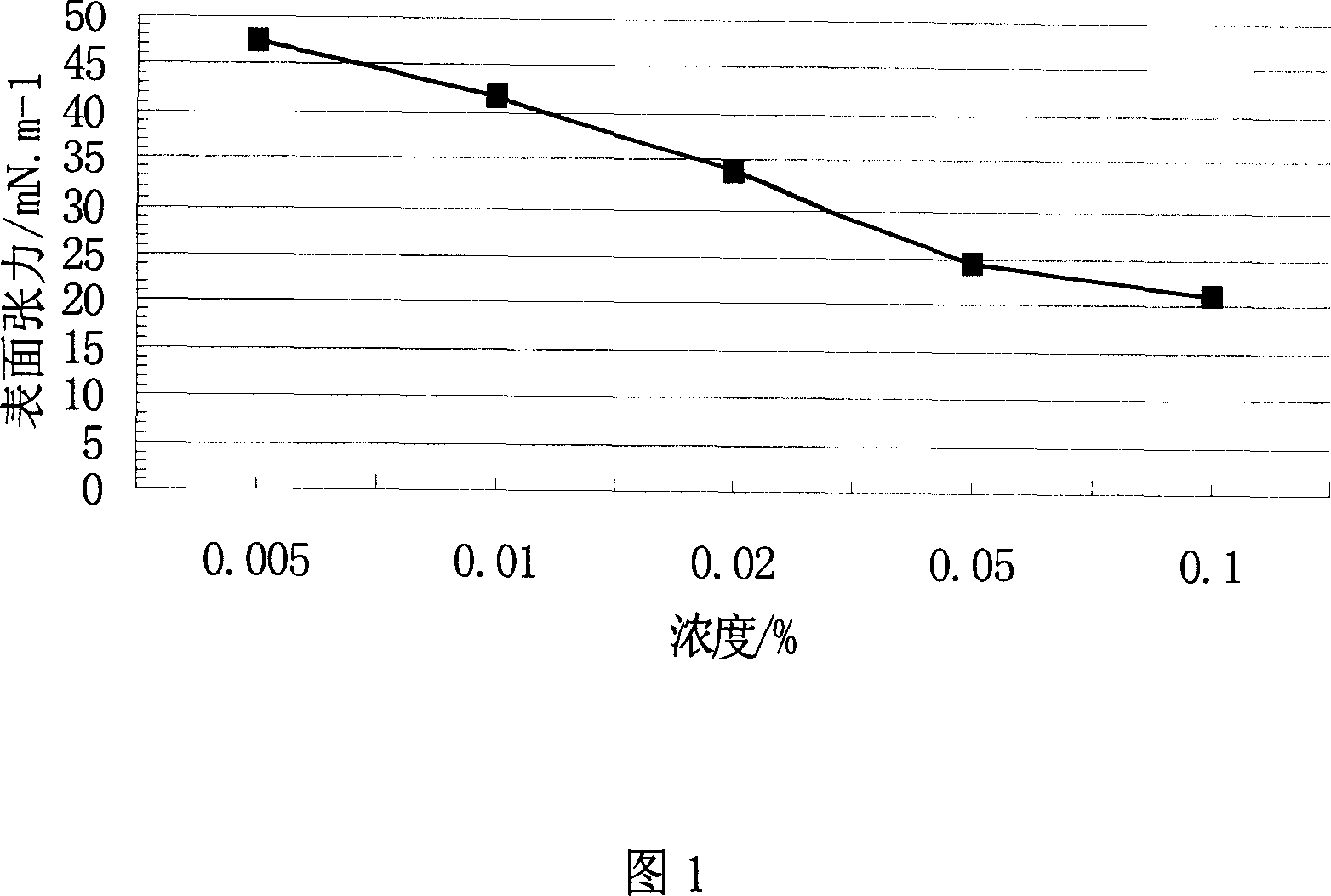
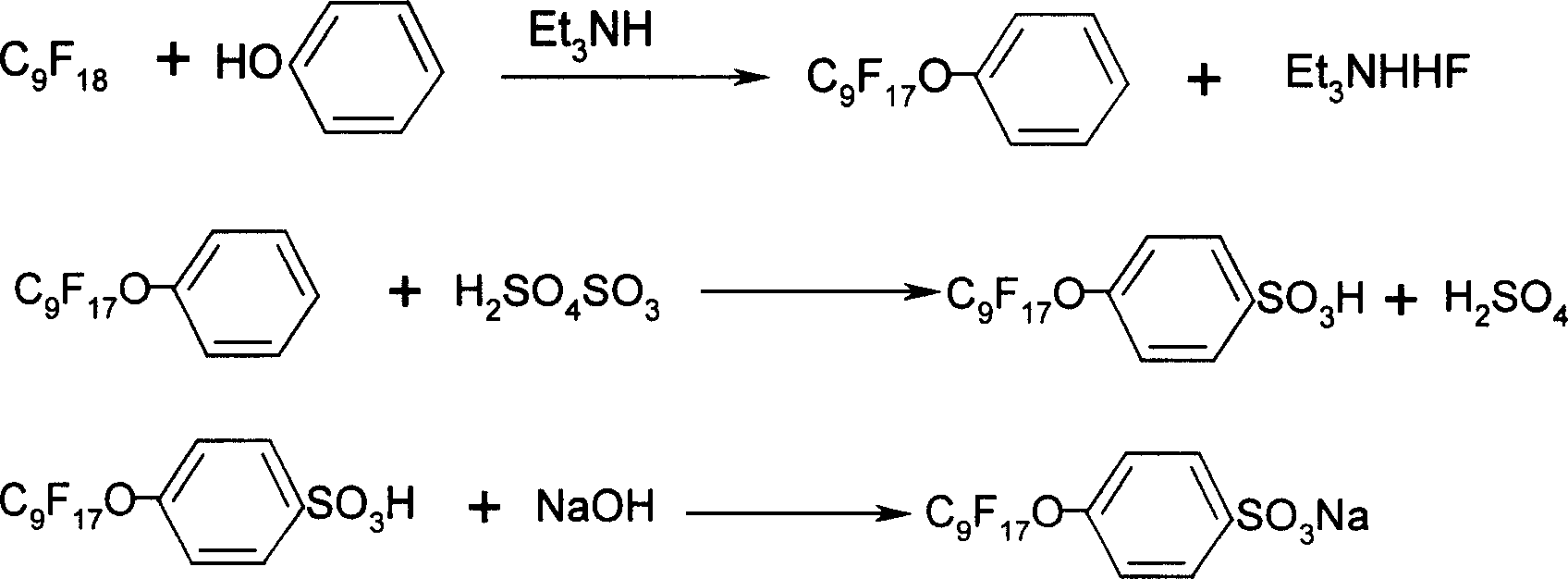
Method of synthesizing sodium p-perfluorous nonenoxybenzenesulfonate
A technology of sodium oxybenzenesulfonate and sodium hydroxybenzenesulfonate, which is applied in the field of synthesis of sodium perfluorononoxybenzenesulfonate, can solve the problems of high product quality, many waste acids, and many process steps, and achieve The effect of short process route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 21g of sodium p-hydroxybenzenesulfonate and 150ml of DMF into a 250ml four-necked glass flask, stir and raise the temperature to 20°C. After dissolving, add 45g of hexafluoropropylene trimer, reflux the system with a condenser tube, and add 14g of triethylamine dropwise. The dropwise addition was completed in about half an hour, and the reaction was continued at the temperature for 3 hours. After cooling, it was poured into 400ml of water, salted out with NaCl, filtered and dried to obtain the product.
Embodiment 2
[0023] Add 21g of sodium p-hydroxybenzenesulfonate into a 250ml four-neck glass flask, fully dissolve it in tetrahydrofuran, stir and raise the temperature to 30°C, after the sodium hydroxybenzenesulfonate is dissolved, add 45g of hexafluoropropylene trimer, and use a condenser tube to Reflux, add 10 g of dimethylamine dropwise, the dropwise addition is completed in about half an hour, continue to maintain the temperature for 3 hours, after cooling, pour into 400ml of water, salt out with NaCl, filter and dry to obtain the product.
Embodiment 3
[0025] Add 21g of sodium p-hydroxybenzenesulfonate into a 250ml four-necked glass flask, fully dissolve it in dimethyl sulfite, stir and raise the temperature to 30°C, after the sodium hydroxybenzenesulfonate is dissolved, add 45g of hexafluoropropylene trimer, and the system Use a condenser to reflux, add 20 g of diamylamine dropwise, and the dropwise addition is completed in about half an hour. Continue to maintain the temperature for 3 hours. After cooling, pour into 400ml of water, salt out with NaCl, filter and dry to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com