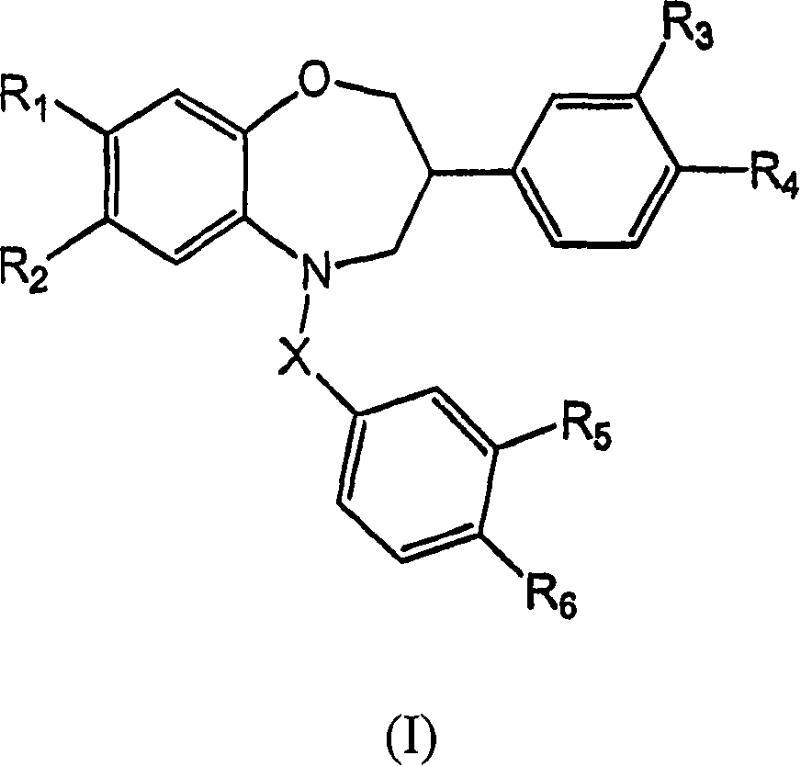
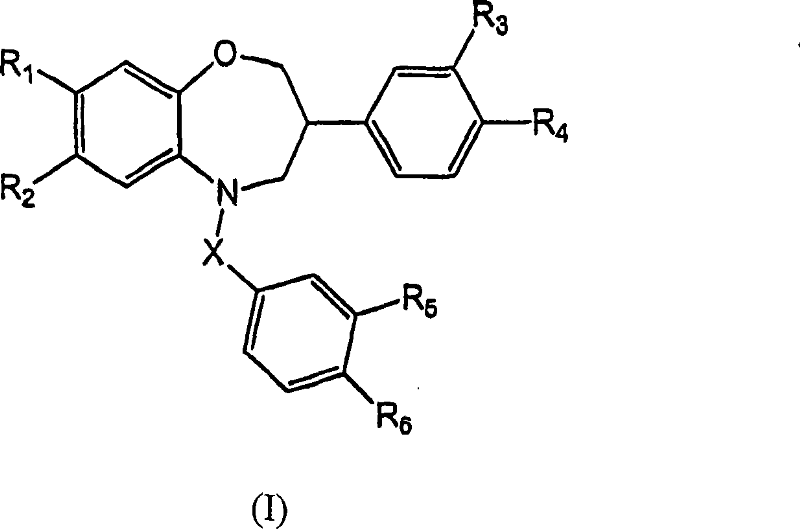
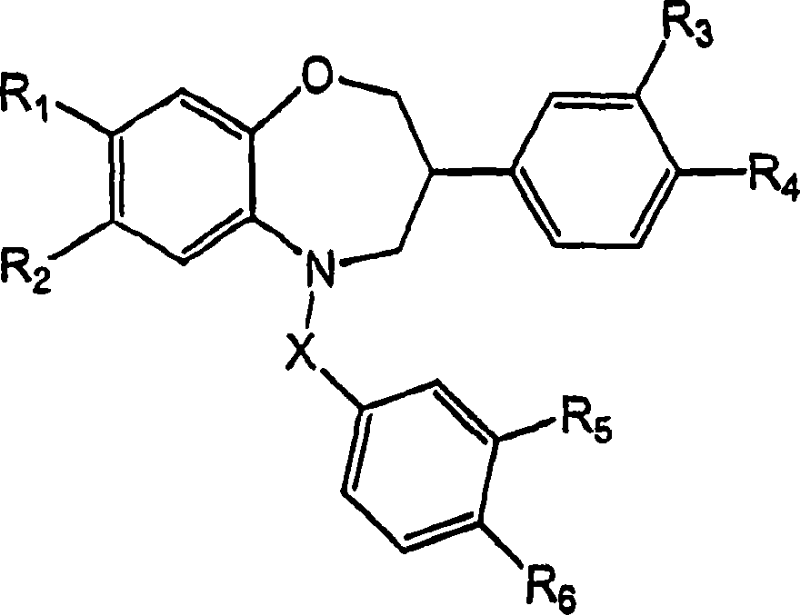
Benzoxazepine derivatives as selective estrogen receptor modulators
A technology of selecting compounds, applied in sexual diseases, drug combinations, organic active ingredients, etc., can solve problems such as exacerbation and inability to improve cognitive function in elderly patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 7-Methoxy-3-(4-methoxy-phenyl)-chromen-4-one
[0067]
[0068] 7-Hydroxy-4'-methoxyisoflavone (25.2 g, 93.9 mmol) was dissolved in a mixture of dry DMF (125 ml) and acetone (125 ml). Anhydrous potassium carbonate (13.0 g, 93.9 mmol) and dimethyl sulfate (8.90 ml, 93.9 mmol) were added successively, and the mixture was heated to 70° C. for 4 hours. After cooling, the reaction mixture was poured on water (1 L). The resulting yellow precipitate was collected by filtration and dried in vacuo to afford the title compound (25.5 g, 96%).
[0069] MS(m / Z)=283(MH+)
Embodiment 2
[0071] 7-Methoxy-3-(4-methoxy-phenyl)-chroman-4-one
[0072]
[0073] 7-Methoxy-3-(4-methoxy-phenyl)-chromen-4-one (13.0 g, 46.0 mmol) was dissolved in anhydrous THF (400 ml) and cooled to -78°C. DIBAL-H (1.5M, 79ml, 0.18mol) was added dropwise over 20 minutes and the reaction was stirred at -78°C for 1 hour. The reaction mixture was quenched with Rochelle's solution (150ml) and stirred at 22°C for 24 hours. The reaction mixture was CH 2 Cl 2 (200ml×2) extraction. Organic layer with H 2 O (500ml x 3) washed, dried over Na2SO4, concentrated in vacuo to afford the title compound (yellow semi-solid) in quantitative yield.
[0074] MS(m / Z)=285(MH+)
Embodiment 3
[0076] 7-Methoxy-3-(4-methoxy-phenyl)-chroman-4-one oxime
[0077]
[0078] A mixture of 7-methoxy-3-(4-methoxy-phenyl)-chroman-4-one (13.14g, 46.22mmol) and hydroxylamine hydrochloride (12.85g, 184mmol) was dissolved In a 1:1 mixture of pyridine and ethanol (20ml), it was heated to reflux under argon atmosphere for 1 hour. The mixture was poured on water (100ml) and the precipitate was collected by vacuum filtration to give the oxime (11.9g, 86%, white solid).
[0079] MS(m / Z)=300(MH+)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com