The application of r-sHSA for treating chronic liver disease
A technology of analogs and substances, applied in the field of recombinant products of a functional gene, can solve the problems of insignificant curative effect, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
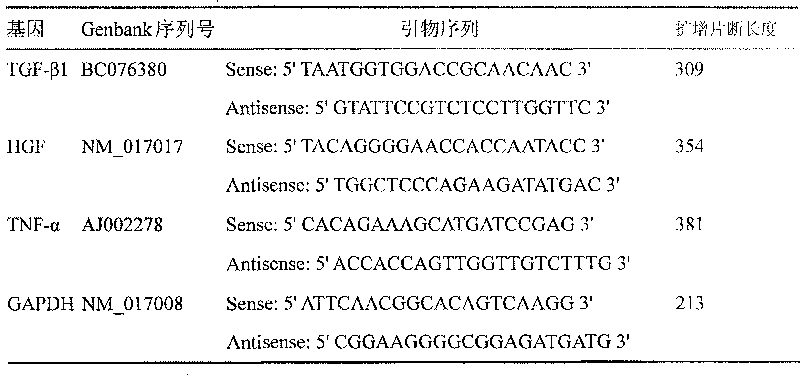
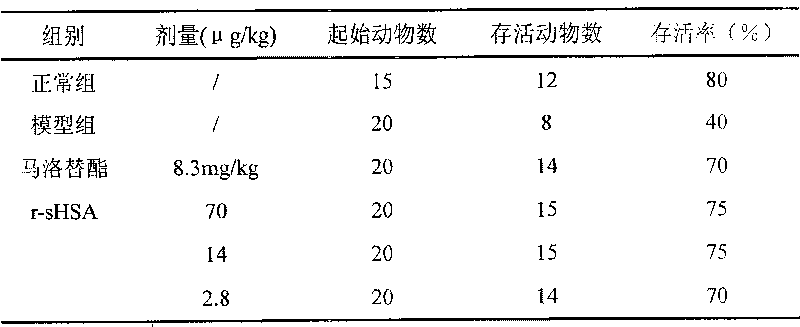
[0024] Protective effect of r-sHSA on carbon tetrachloride-induced liver fibrosis in rats
[0025] 1. Test drugs
[0026] Drug dosage: r-sHSA high dose group is 70μg / kg, the prepared concentration is 7μg / ml, i.p0.1ml / 10g; the middle dose group is 14μg / kg, prepared concentration is 1.4μg / ml, i.p0.1ml / 10g ; The low dose group is 2.8μg / kg, the preparation concentration is 0.28μg / ml, i.p0.1ml / 10g.
[0027] Experimental control: The blank control was injected intravenously with physiological saline in the same volume as the administration group.
[0028] Positive control: malotilate. Before use, add distilled water as needed to make a concentration of 0.83mg / ml, suspend evenly, and store at 4°C for later use.
[0029] 2. Experimental animals
[0030] SPF grade Wistar rats, all male, weighing 160-180g.
[0031] 3. Other experimental materials
[0032]Analytical pure carbon tetrachloride; phenobarbital sodium injection (0.1g / bottle); Luhua brand pure peanut oil; sodium chloride...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com