Functional group linken on surface of polymer electrolyte nano SiO2 and preparation method thereof
A surface functional group and electrolyte technology, which is applied in the treatment of dyed low-molecular organic compounds, secondary battery parts, fibrous fillers, etc., can solve the problems of difficult to achieve reaction conditions, affect yield, and low yield, so as to improve electrical conductivity rate, reducing the effect of crystal phase content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
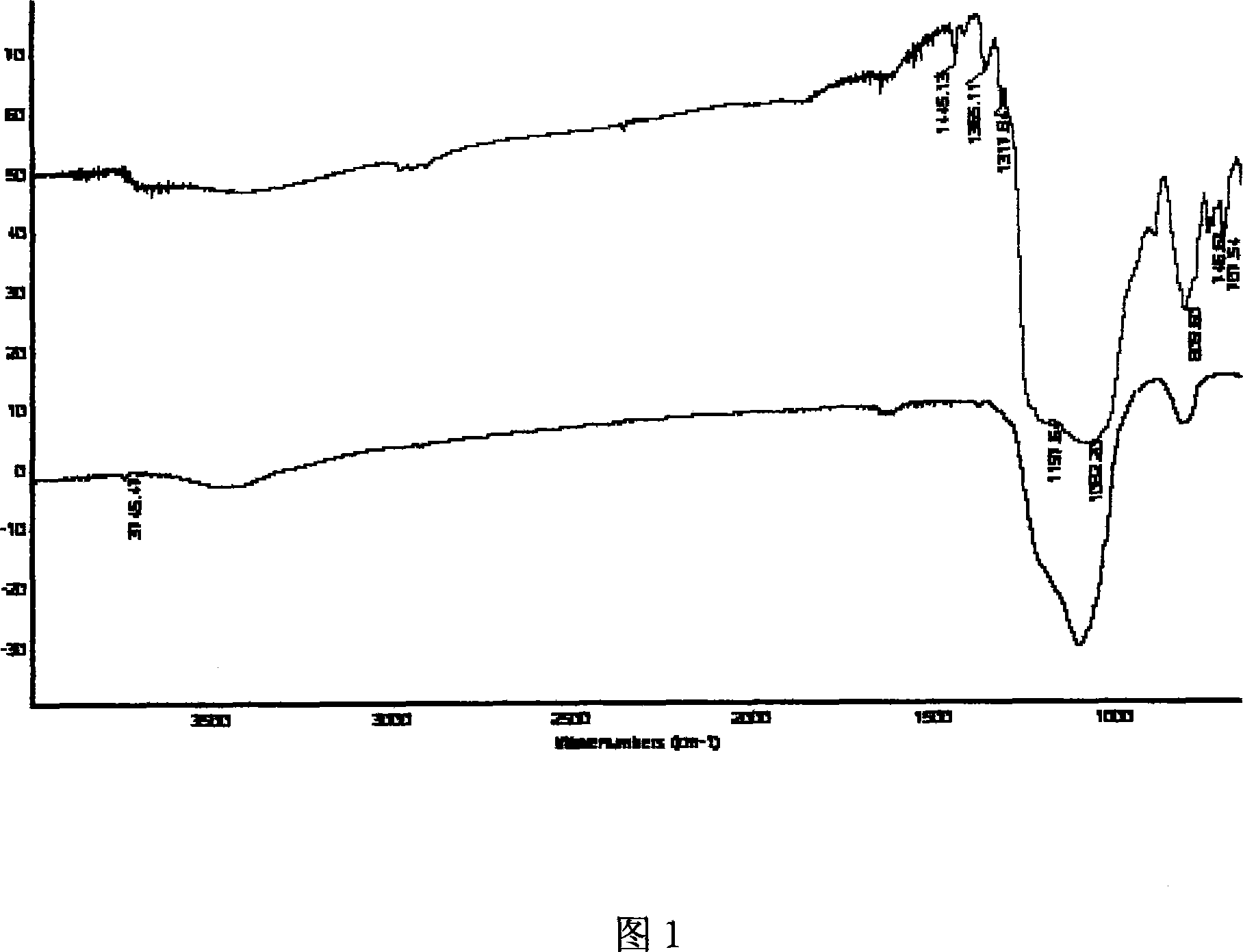
[0025] Embodiment 1. Get 10g of original sintered, particle size is 7~20nm, specific surface area is 380m 2 / g nano-SiO 2 , placed in a muffle furnace at 450°C for pre-burning for 24 hours, then transferred to 30ml of 5M HCl solution for pickling, and stirred at room temperature for 3 hours. The pickled SiO 2 Filter, wash, and dry under vacuum at 120°C for 12 hours. Weigh 5g of treated SiO 2 , placed in an airtight container under the protection of an inert gas, while adding 2g of silane coupling agent Cl 3 Si-CH 2 CH 2 (CF 2 ) 5 CF 3 and 0.5g catalyst HN(CH 3 ) 2 , and finally add 30ml CH 2 Cl 2 solvent. The mixture was stirred and heated at 45-55°C for 10-12 hours. After the reaction, the mixture was filtered in a fume hood, followed by CH 3 CH 2 OCH 2 CH 3 , anhydrous CH 3 CH 2OH wash. Then put the surface-modified nanoparticles into a vacuum oven and bake at 120°C. Figure 1 shows the nano-SiO 2 Diffuse reflectance infrared spectra before and after mo...
Embodiment 2
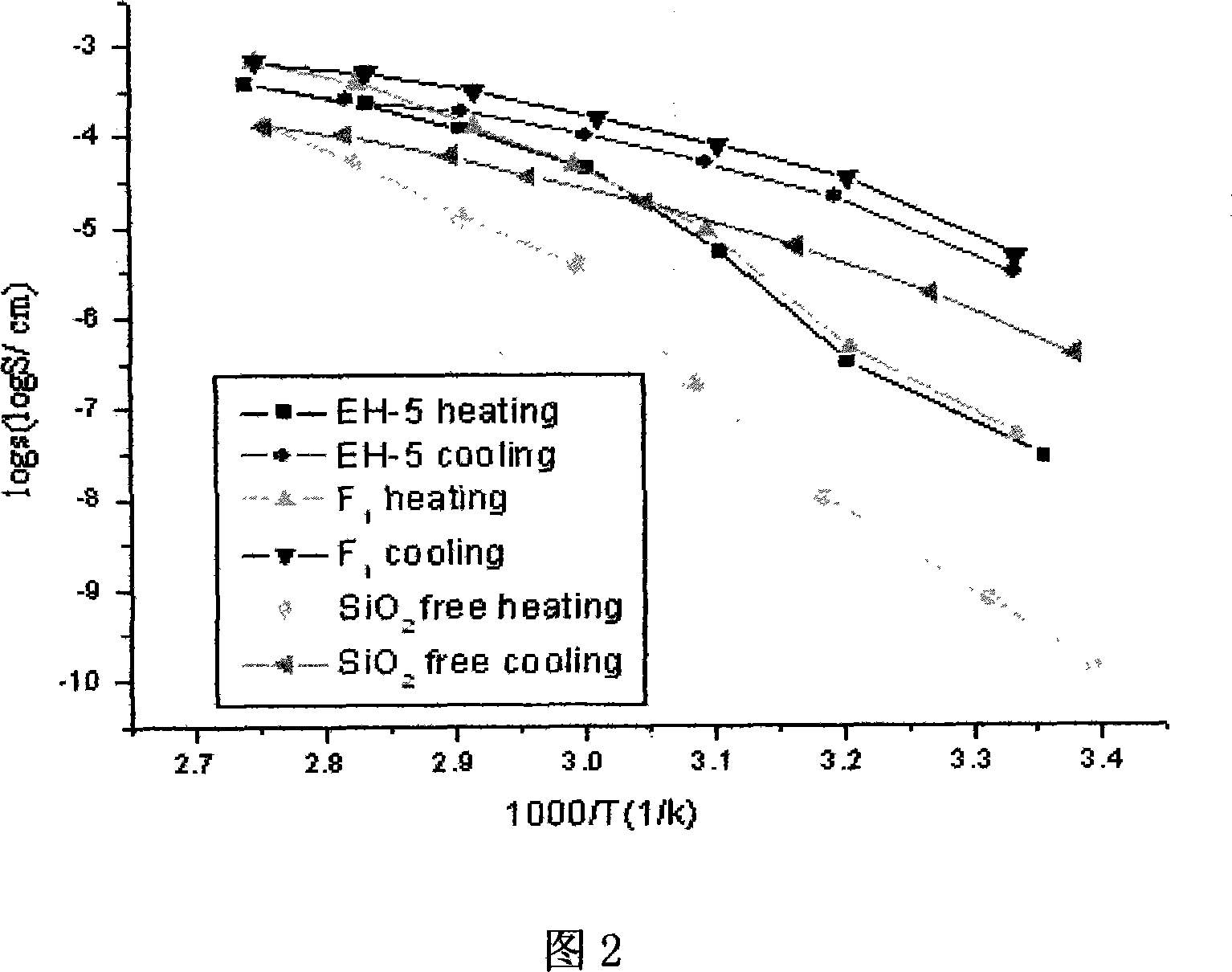
[0026] Embodiment 2. Weigh 0.7gPEO (weight average molecular weight~600,000, the same below), 0.2108gLiClO 4 , and then put them together in a dry round bottom flask, add 40ml of acetonitrile (CH3CN, HPLC) as a solvent, under the protection of an inert gas, control the temperature to 50 ° C, and stir for 60 hours. After the mixture becomes a sol, cast it on a polytetrafluoroethylene template, absorb the volatilized solvent with a 4 Å molecular sieve, transfer it to a vacuum drying oven after 24 hours, and continue drying at 60°C for 48 hours. This results in a dry, uniform, self-supporting pure polymer electrolyte membrane.
Embodiment 3
[0027] Embodiment 3. the original sintered nano-SiO before modification 2 Baked in vacuum at 120°C for 24 hours, weighed 0.09108g, mixed with 0.7gPEO, 0.2108g LiClO 4 Put them together into a dry round bottom flask, add 40ml of acetonitrile (CH3CN, HPLC) as a solvent, under the protection of an inert gas, control the temperature at 50°C, and stir for 60 hours. After the mixture becomes a sol, cast it on a polytetrafluoroethylene template, absorb the volatilized solvent with a 4 Å molecular sieve, transfer it to a vacuum drying oven after 24 hours, and continue drying at 60°C for 48 hours. This results in a dry, uniform, self-supporting composite polymer electrolyte membrane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com