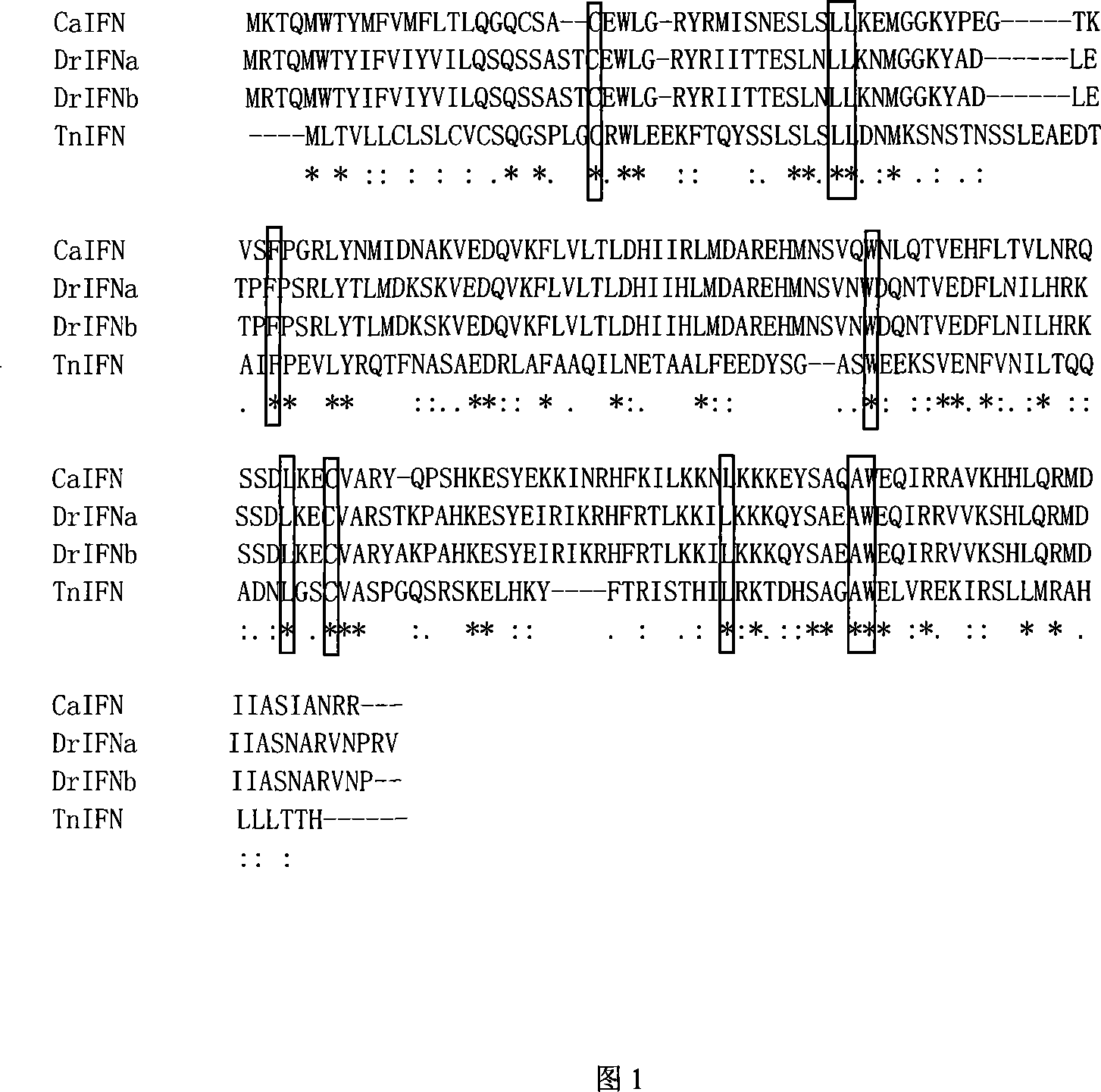
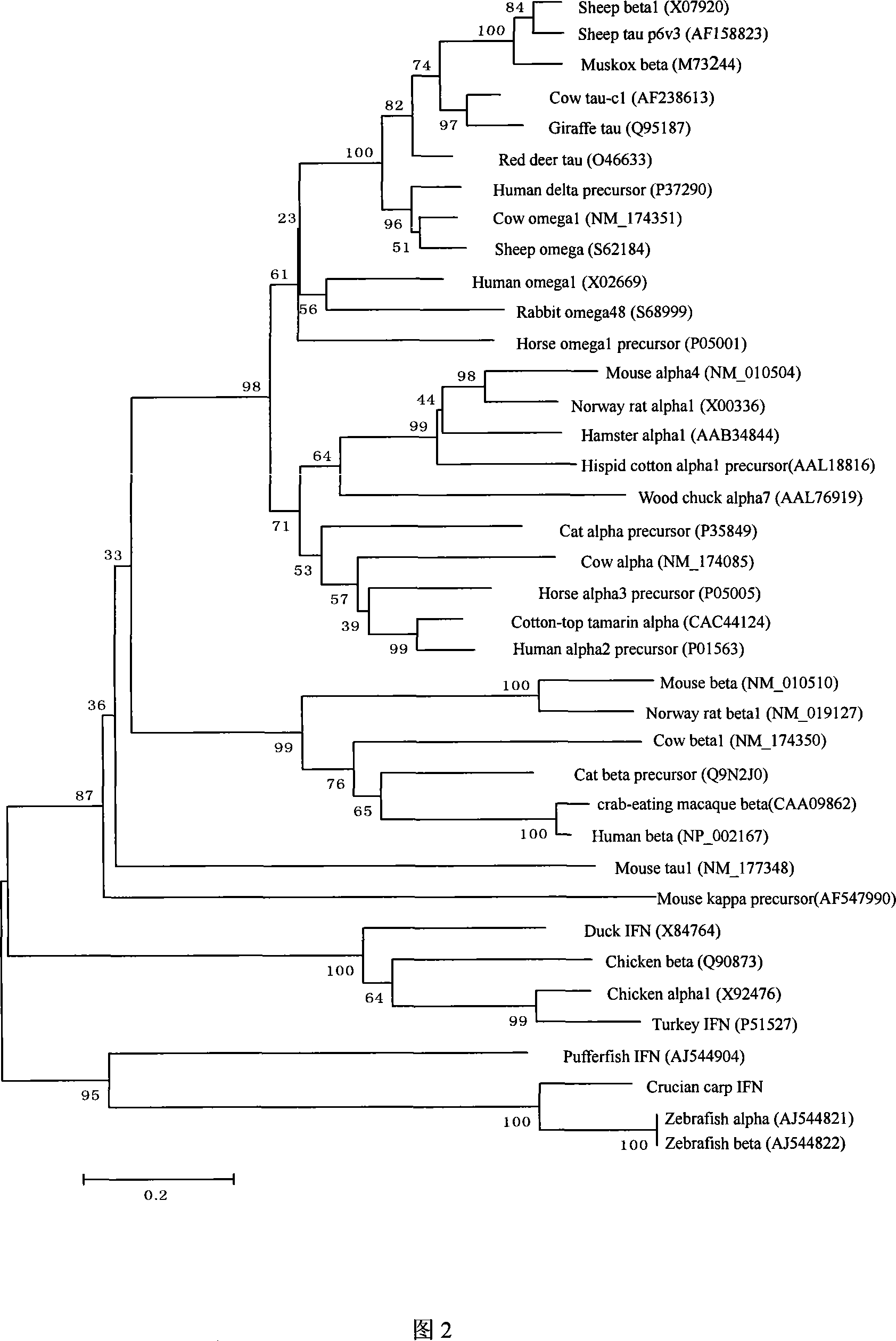
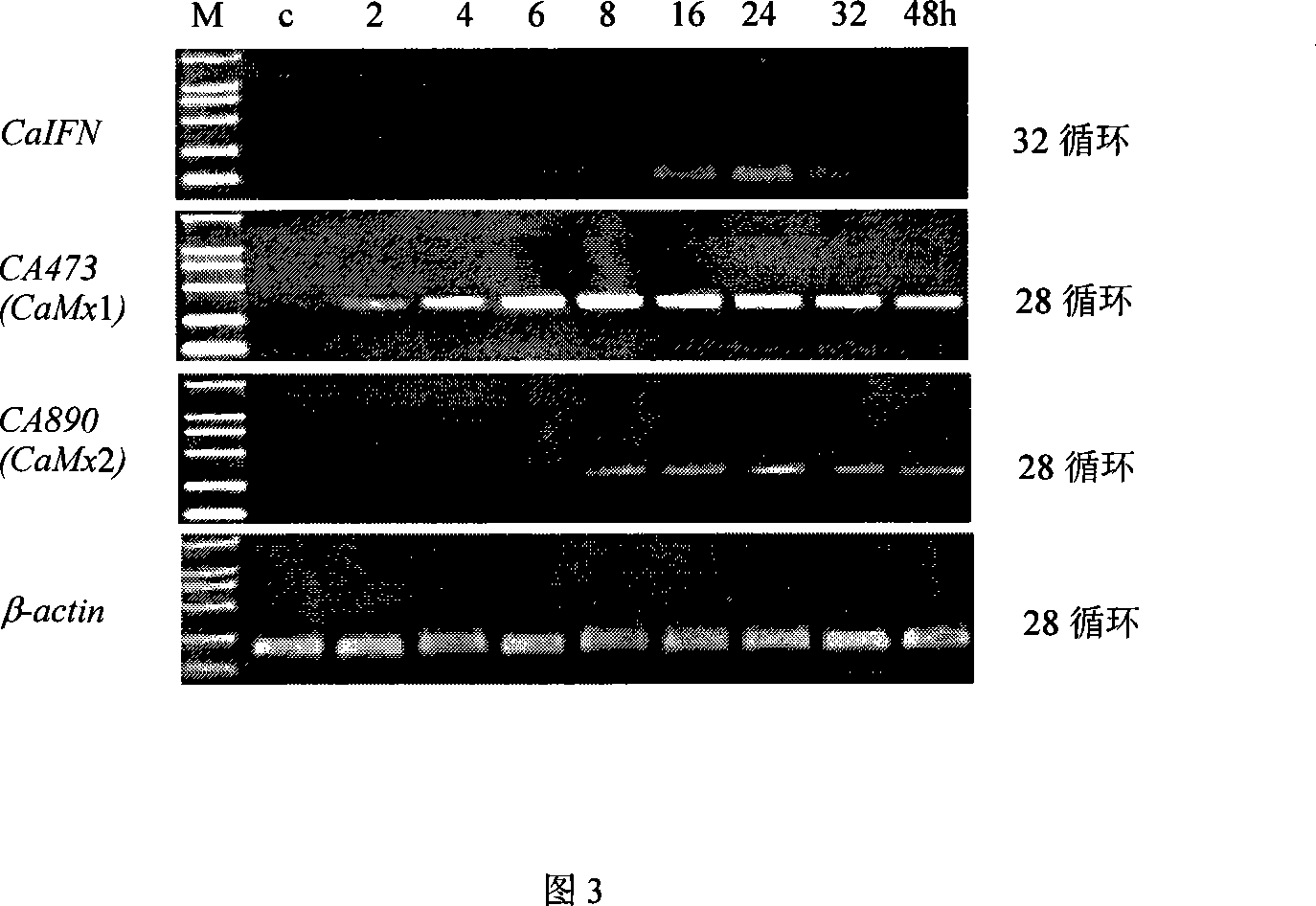
Fish interferon gene and use thereof
A technology of interferon and fish, applied in the field of genetic engineering, can solve the problem that the research and application of fish interferon have not made great progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Virus inactivation and induction of CAB interferon in crucian carp blastula cells
[0035] Crucian carp blastula cell line (CAB) and grass carp kidney cell line (CIK) were conventionally cultured at 28°C in 199 medium (GIBCO) with 10% newborn bovine serum (inactivated at 56°C for 30 min before use). GCHV was amplified on CIK cells and virus titers were detected. GCHV was purified by differential centrifugation before inactivation by UV light: 4×10 3 g centrifugation for 20 min to remove cell residues, and the supernatant was subjected to 1×10 5 g ultracentrifugation for 1.5h to precipitate the virus, and the virus pellet was diluted with an appropriate amount of serum-free 199 medium, and continued to 1.5×10 3 g centrifuged for 20min to get the supernatant to obtain purified virus. The inactivation device uses a 30W ordinary ultraviolet germicidal lamp, fixed on the ultra-clean workbench, and the vertical distance from the shaker is 16cm. Before inactivat...
Embodiment 2
[0037] Example 2: Total RNA extraction and mRNA purification of virus-infected fish cells
[0038] Total RNA was extracted from CAB cells induced by inactivated GCHV and control CAB cells without virus induction under the same conditions by ultracentrifugation with CsTFA (cesium trifluoroacetate, Pharmacia). After the cells were induced by the virus, they were washed 3 times with PBS. Then, 4 mL of extraction buffer was added to each bottle of cells to disrupt the cells, and the cells were centrifuged at 5000 g at 15°C for 20 min to remove cell residues. The supernatant was aspirated 10 times with a No. 7 needle, and then ultracentrifuged. During ultracentrifugation, add 6.65mL CsTFA to 6 14×89mm centrifuge tubes (3 each for experimental cells and 3 control cells), add about 3.5mL of extract sample to each tube, 125000g, 15°C, use SW41 The head was ultracentrifuged for 16h. After centrifugation, add 100 μL of TE (10 mM Tris-Cl, pH 7.51 mM EDTA) to each centrifuge tube to diss...
Embodiment 3
[0040] Example 3: Synthesis of fish cell SMART cDNA infected with virus
[0041] Carry out according to the experimental procedures established in the manual of Clontech SMART PCR Synthesis Kit. The steps are: 1 μg PolyA + RNA was mixed with cDNA synthesis primer (CDS primer) and 1 μL of SMART II oligonucleotide primer, and water was added to a final volume of 5 μL. The PCR instrument was placed on ice at 72°C for 2 minutes, and then 2 μL of 5× first-strand reaction buffer was added. , DTT 1μL, 50×dNTP 1μL, PowerScript reverse transcriptase 1μL, and incubated at 42°C for 1h to synthesize the first-strand cDNA. Then take 2 μL in 100 μl system for second strand synthesis by LD-PCR. The PCR reaction parameters were: pre-denaturation at 95°C for 1min, followed by 13 cycles of 95°C for 5s, 65°C for 5s, and 68°C for 6min. The PCR product can be used for RACE-PCR to amplify the full-length cDNA of the specifically expressed gene. This experiment successfully obtained the full-len...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body length | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| body length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com