Method of synthesizing ifosfamide
A technology of ifosfamide and its synthesis method, which is applied in the fields of chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., and can solve problems such as complex post-processing, inconvenient industrial production, and long reaction time , to achieve the effect of easy industrial production, convenient source and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
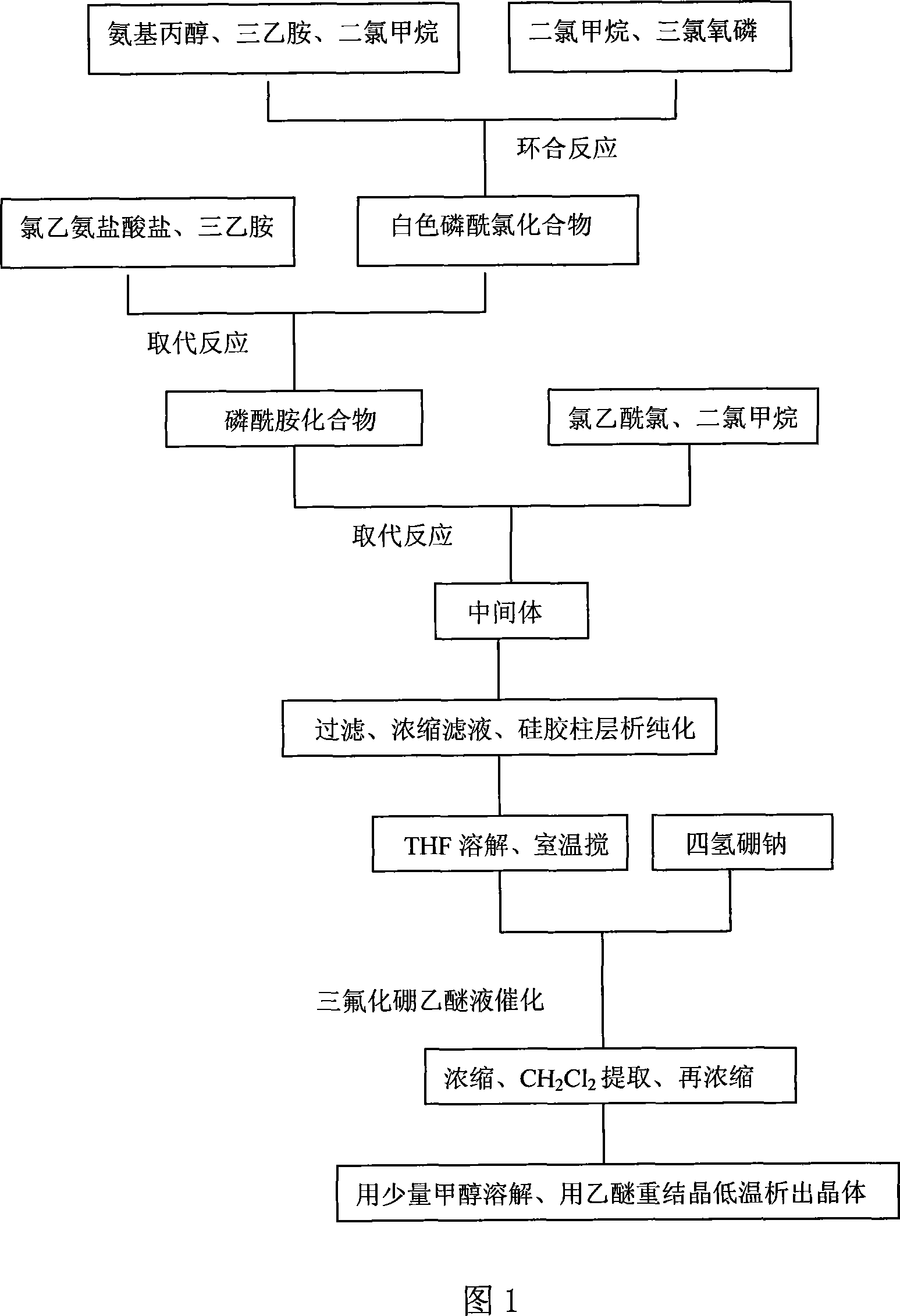
[0033] 1. Preparation of intermediate 3-(2-chloroacetyl)-2-[(2-chloroethyl)amino]-tetrahydro-2H-1,3,2-oxaphosphorus-2-oxide:
[0034] Add a dichloromethane solution of 0.068mol / 30ml of phosphorus oxychloride to a 250ml four-necked flask, cool with ice water, and add dropwise a mixed solution of 0.066mol of aminopropanol, 0.134mol of triethylamine and 10ml of dichloromethane, and stir thoroughly , A large amount of white matter is generated. After the dropwise addition, the reaction was carried out at -5°C for 2 hours. Then 0.062 mol of chloroethylamine hydrochloride was added to the reaction solution, and then 0.124 mol of triethylamine was added dropwise to complete the reaction in about 3 hours. Cool in an external ice bath, slowly add dropwise a dichloromethane solution of 0.062mol / 60ml of chloroacetyl chloride, and control the internal temperature at -5°C, and continue the reaction for 5 hours after the dropwise addition is completed. The triethylamine hydrochloride was remove...
Embodiment 2
[0047] 1. Preparation of intermediate 3-(2-chloroacetyl)-2-[(2-chloroethyl)amino]-tetrahydro-2H-1,3,2-oxaphosphorus-2-oxide:
[0048] Add phosphorus oxychloride in methylene chloride solution 13.1mol / 6000ml into a 50L reactor, cool the ice-water mixture, and slowly add a mixed solution of 13.2mol aminopropanol, 26.7mol triethylamine and 2000ml methylene chloride, and stir thoroughly , A large amount of white matter is generated. After the addition is complete, react at room temperature 25°C for 8 hours. Then 13.0 mol of chloroethylamine hydrochloride was added to the reaction solution, and then 25.1 mol of triethylamine was slowly added to complete the reaction, and the reaction was completed in about 5 hours. Cool outside with ice water, slowly add 12.5mol / 12000ml of dichloromethane solution of chloroacetyl chloride and control the inner temperature at 20°C. After completion, the reaction was continued for 10 hours. The triethylamine hydrochloride was removed by filtration, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com