Catalyst for carbon monoxide oxidation in hydrogen and preparation method thereof
A carbon monoxide and catalyst technology, which is applied to a catalyst for selective oxidation of carbon monoxide in hydrogen at low temperature and its preparation field, can solve the problems of high raw material price, reduced fuel utilization efficiency, low catalyst selectivity, etc., and achieves low price, reduced preparation cost, and improved The effect of energy efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
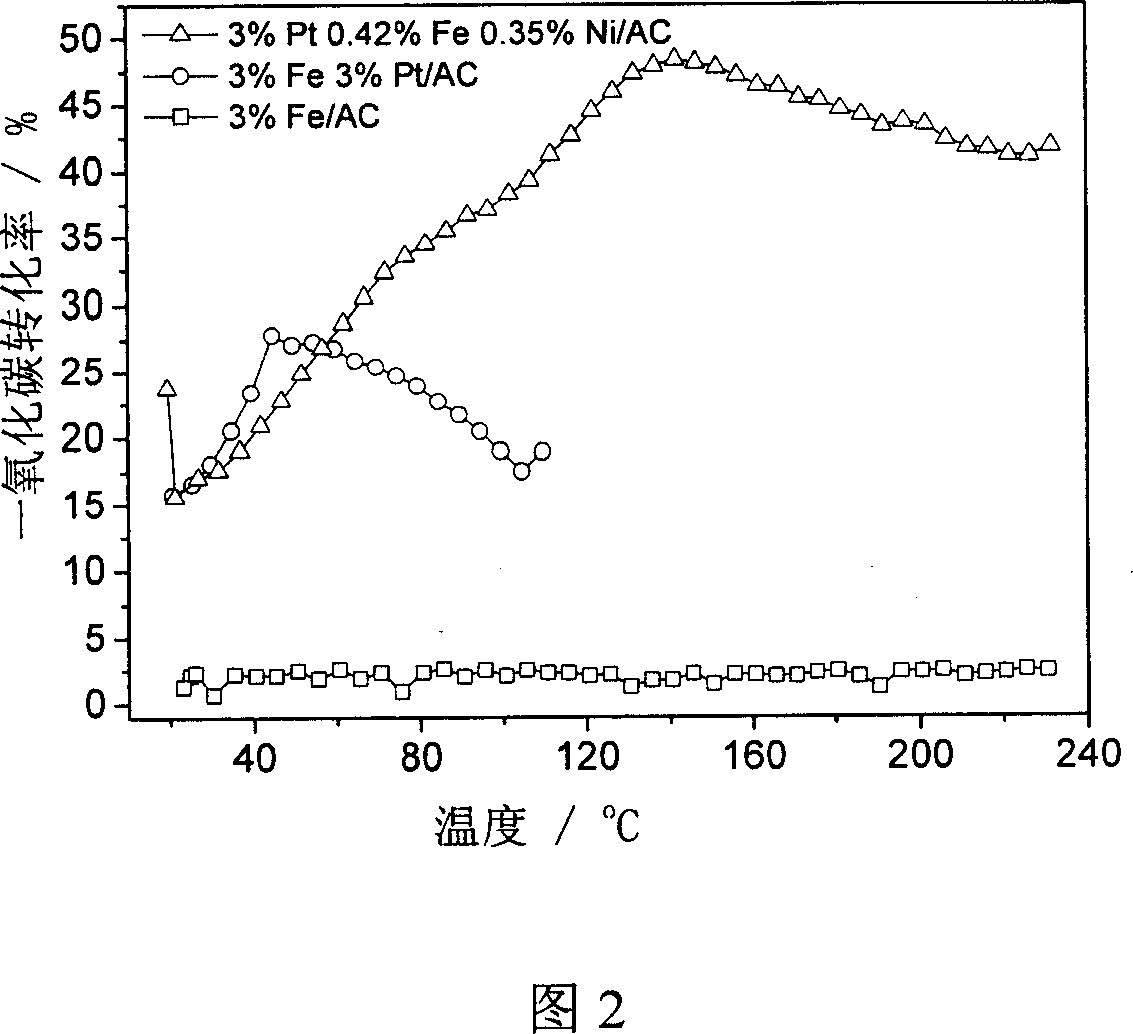
[0026] The silver catalyst supported by active carbon is prepared by equal volume impregnation method, and the silver nitrate of 0.189g is dissolved in 3 milliliters of deionized water according to the total amount ratio of active component and carrier, and 1 gram of active carbon carrier is added thereto, placed at room temperature after stirring Dry at 60° C. for two days to obtain a supported catalyst with a silver loading amount of 12%. After it was treated at 500°C under raw material gas for 2 hours, the active components mainly existed in the form of metallic silver.
Embodiment 2
[0028] The catalyst is prepared by impregnation method using chloroplatinic acid hexahydrate as precursor and carbon black as carrier. Take by weighing 1.048g of an aqueous solution with a platinum concentration of 1.909%, add 6 milliliters of ethanol and 7 milliliters of ionized water, then add 1g of carbon black therein, leave it at room temperature for two days after stirring, and dry at 120°C to obtain the loading capacity of platinum. 2% loaded catalyst. The catalyst was treated with hydrogen at 500°C for 2 hours before the reaction.
Embodiment 3
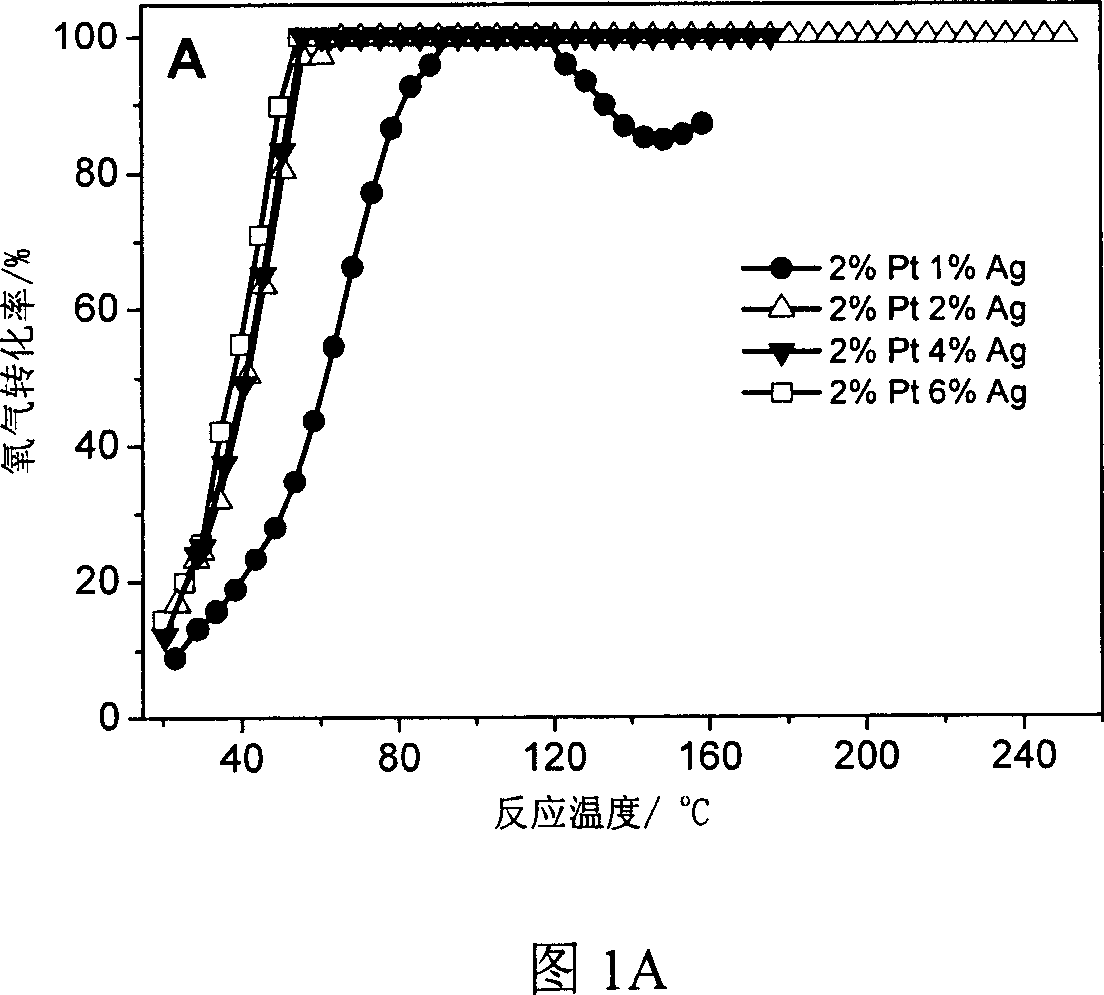
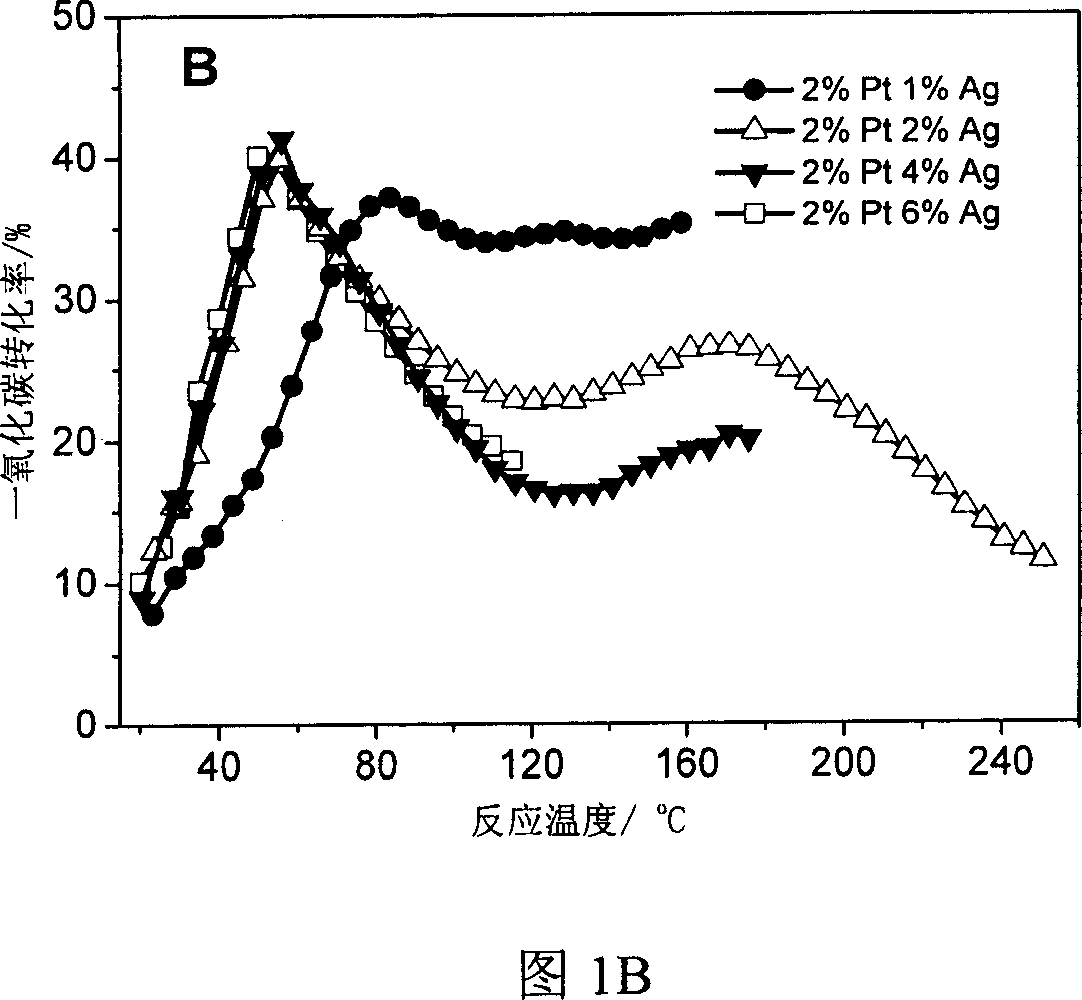
[0030] According to the weight ratio of silver and platinum, weigh a certain amount of aqueous solution with a platinum concentration of 1.909%, add 1g of carbon black to it, stir and place it at room temperature for two days, dry it at 120°C, and then impregnate 0.094g of silver nitrate aqueous solution , drying at 120°C. The catalyst was treated with hydrogen at 500°C for 2 hours before the reaction. The mass ratio of silver to platinum is 6:2, 1:2, 2:2, 2:3. Bimetallic silver and platinum catalysts can be impregnated in stages, such as first impregnating silver and then impregnating platinum, or first impregnating platinum and then impregnating silver, or co-impregnating. Experiments have confirmed that the catalyst prepared by impregnating platinum first and then silver has good catalytic activity. .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com