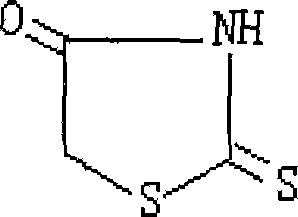
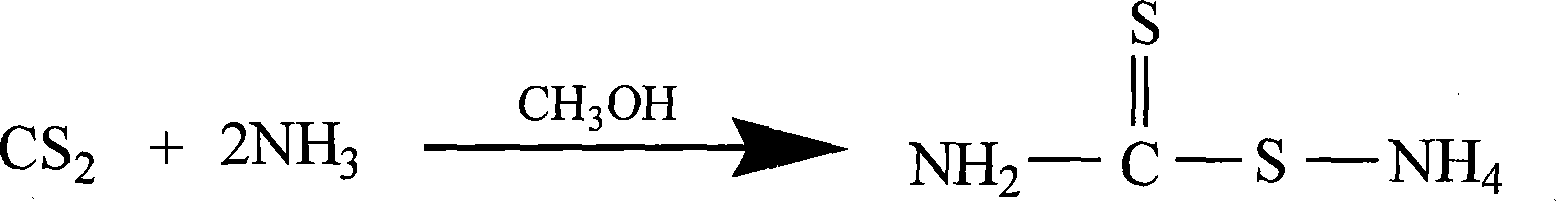
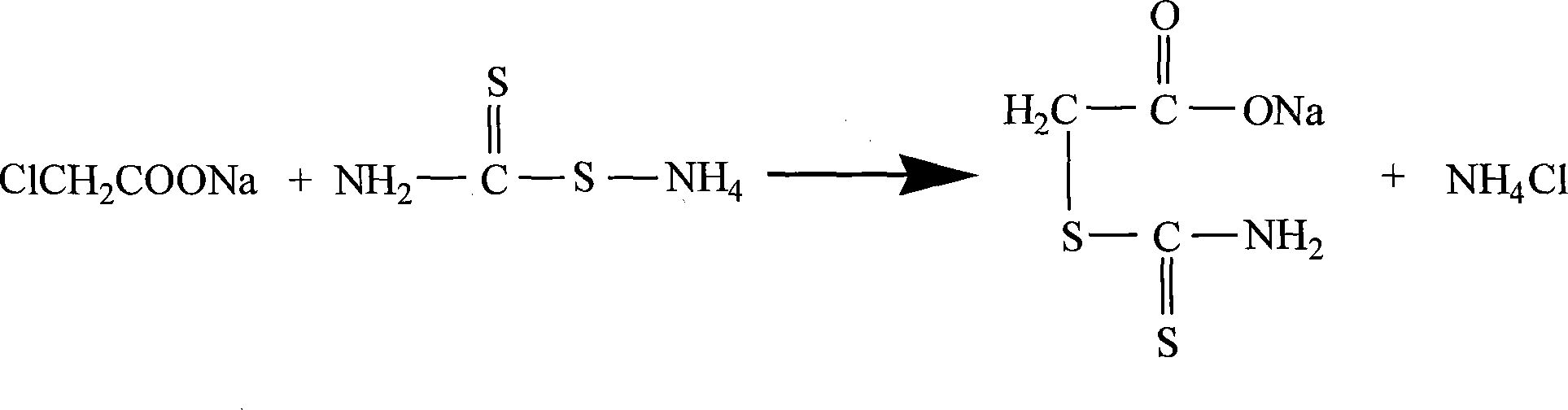
Prepn process of 2-thiothiazolidone
A technology of thiothiazolidinone and chlorination, which is applied in the field of preparation of 2-thiothiazolidinone, can solve the problems such as the preparation method of no substituent-free rhodanine, which is beneficial to health and reduces the volatilization of raw materials , the effect of improving the working environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Synthesis of ammonium dithiocarbamate: Introduce ammonia gas into methanol until it becomes a saturated solution, cool the ammonia-methanol solution and carbon disulfide to 4°C respectively, put 155ml of cold carbon disulfide in the reaction kettle, and add ammonia dropwise under stirring 500ml of methanol solution, the rate of addition was controlled to keep the reaction system at 4-6°C, and stirred for 1.5 hours after the addition was completed. Stand at room temperature for 10 hours, and filter to obtain 210 g of ammonium dithiocarbamate as a solid.
[0024] 2. Condensation: Add the above solids to the sodium chloroacetate solution made up of 360g chloroacetic acid, 150g sodium hydroxide and 1440ml pure water, stir and react under cooling at 15-20°C for 30 minutes, filter to obtain a clear condensation liquid.
[0025] 3. Cyclization: Add the hydrochloric acid solution with a concentration of 4N into the reaction kettle, slowly add the above-mentioned condensatio...
Embodiment 2
[0028] 1. Synthesis of ammonium dithiocarbamate: Cool the saturated ammonia ethanol solution and carbon disulfide to 4°C respectively, put 155ml of cold carbon disulfide in the reaction kettle, add 1200ml of ammonia ethanol solution dropwise under stirring, control the rate of addition, and make the reaction The system was kept at 4-10°C, and stirred for 2.5 hours after the drop was completed. Stand at room temperature for 10 hours, and filter to obtain 196 g of ammonium dithiocarbamate as a solid.
[0029] 2. Condensation: Add the above solids to the sodium chloroacetate solution made up of 350g chloroacetic acid, 150g sodium hydroxide and 1440ml pure water, stir and react under cooling at 15-20°C for 20 minutes, and filter to obtain a clear condensation liquid.
[0030] 3. Cyclization: Add the sulfuric acid solution with an equivalent concentration of 5N into the reaction kettle, slowly add the above-mentioned condensation solution under stirring, and then raise the tempera...
Embodiment 3
[0033] 1. Synthesis of ammonium dithiocarbamate: Cool the saturated ammonia isopropanol solution and carbon disulfide to 4°C respectively, put 155ml of cold carbon disulfide in the reaction bottle, add 1200ml of ammonia isopropanol solution dropwise under stirring, and control the dropwise Acceleration, keep the reaction system at 4-10°C, and stir for 1 hour after the dropwise completion. Stand at room temperature for 10 hours, and filter to obtain 224 g of ammonium dithiocarbamate as a solid.
[0034] 2. Condensation: Add the above-mentioned solids to the sodium chloroacetate solution made of 383g chloroacetic acid, 160g sodium hydroxide and 1500ml pure water at 15-20°C, stir for 60 minutes, and filter to obtain a clear condensation liquid.
[0035] 3. Cyclization: add the hydrochloric acid solution with an equivalent concentration of 6N into the reaction kettle, slowly add the above-mentioned condensation solution under stirring, and then raise the temperature to 90°C for 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com