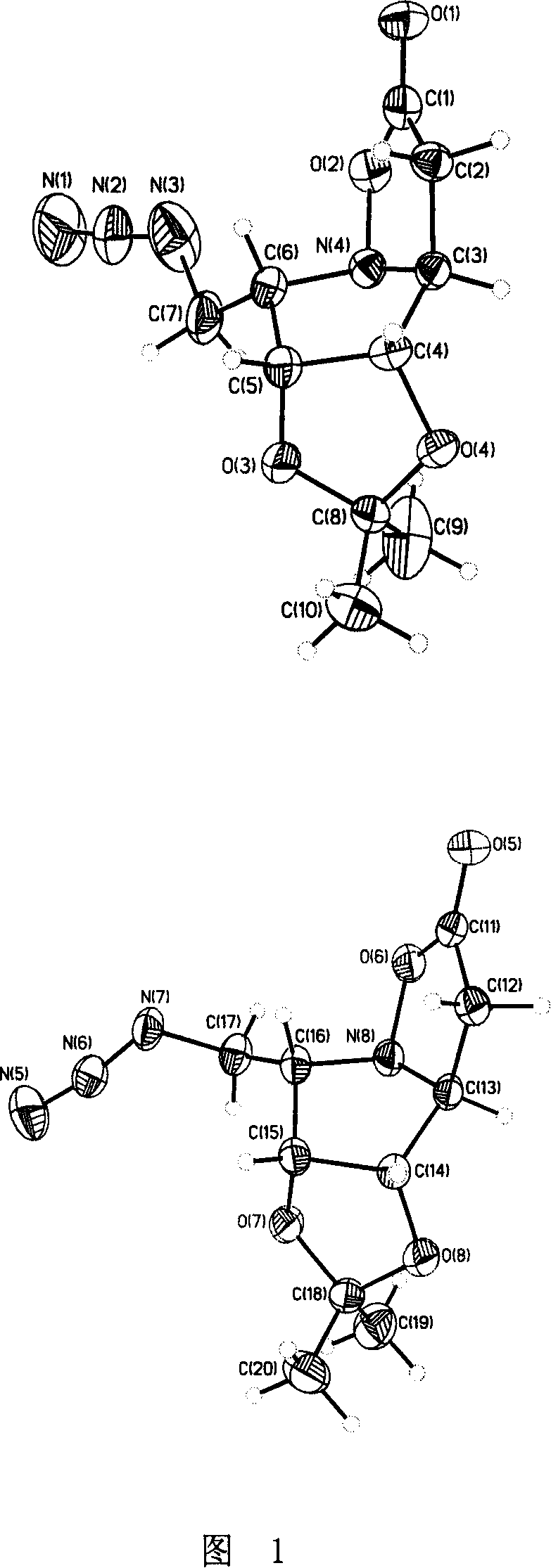
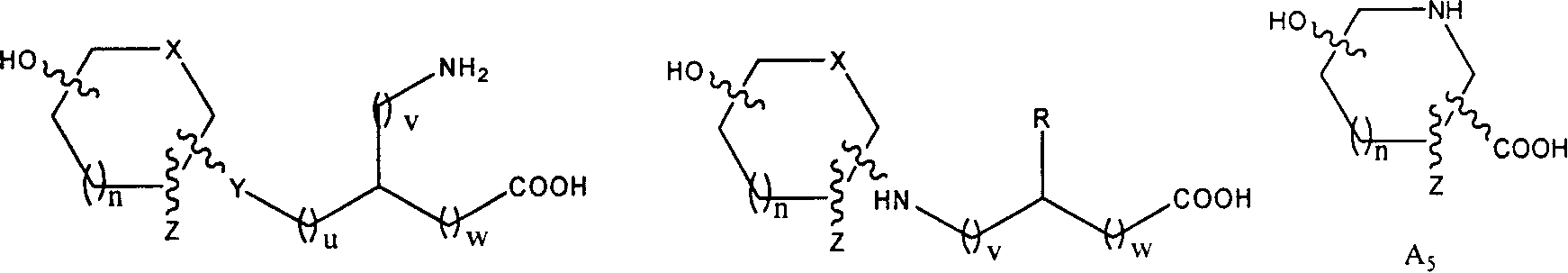
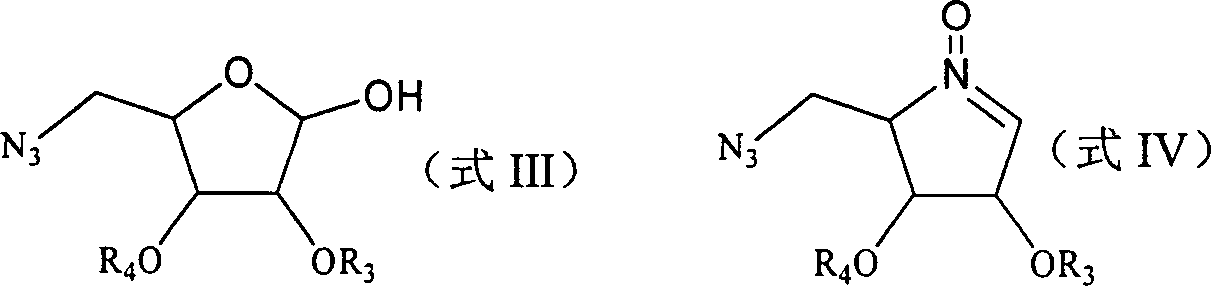Dicarboxy Boc-L drivative, its preparing method and use
A high-proline, double-hydroxyl technology, applied in organic chemistry and other fields, can solve the problems of not reaching the level of automation of peptide synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Embodiment 1: the synthesis of sugar oxime (compound 2)
[0099]
[0100] 5-deoxy-5-azido-2,3-O-isopropylidene-D-ribofuranose (1) (4.0 g, 18.6 mmol) was dissolved in 20 ml of dichloromethane, and pyridine was added at room temperature ( 5.9 ml, 74.4 mmol), hydroxylamine hydrochloride (2.6 g, 37.2 mmol), reacted under stirring for 12 hours, TLC showed that the reaction was complete. Add 20 milliliters of water to the reaction system, separate the liquids, extract the aqueous phase with 20 milliliters of ethyl acetate three times, combine the organic phases, dry the organic phases with anhydrous sodium sulfate, filter after drying, concentrate the filtrate under reduced pressure and separate by column chromatography (eluent: petroleum ether: ethyl acetate = 4:1), and 4.2 g of the product sugar oxime (compound 2) was obtained. The product was a white solid with a yield of 98%.
[0101] R f =0.44 (petroleum ether: ethyl acetate=2:1).
[0102] Melting point: 91-92℃. ...
Embodiment 2
[0107] Embodiment 2: the synthesis of the sugar oxime (compound 3) of TBS protection
[0108]
[0109] (2) (4.2 g, 18.2 mmol) was dissolved in 20 ml of dichloromethane, tert-butyldimethylsilyl chloride (4.3 g, 28.7 mmol), pyridine (3.8 ml, 47.5 mmol) were added successively at room temperature mol), after reacting under stirring for 24 hours, TLC showed that the reaction was complete. Add 20 milliliters of water to reaction system, separate liquid, water phase is extracted 3 times with 20 milliliters of dichloromethanes, combine organic phase, filter after organic phase is dried with anhydrous sodium sulfate, after the filtrate is concentrated under reduced pressure, separate through column chromatography (washing Removal agent: petroleum ether: ethyl acetate = 8: 1), to obtain 5.1 g of the product TBS-protected sugar oxime (compound 3). The product was a colorless syrup with a yield of 81%.
[0110] R f =0.78 (petroleum ether: ethyl acetate=4:1); R f =0.78 (petroleum e...
Embodiment 3
[0115] Embodiment 3: the synthesis of mesylate sugar oxime (compound 4)
[0116]
[0117] (3) (5.0 g, 14.5 mmol) was dissolved in 30 ml of dichloromethane, methanesulfonyl chloride (1.7 ml, 29.5 mmol) was added under ice cooling, triethylamine (4.1 ml, 29.5 millimoles), the dropwise addition was complete, and TLC showed that the reaction was complete. Add 30 milliliters of ice-water to reaction system, separate liquid, water phase is extracted 3 times with 30 milliliters of dichloromethane, combine organic phase, filter after organic phase is dried with anhydrous sodium sulfate, after the filtrate is concentrated under reduced pressure, separate through column chromatography ( Eluent: petroleum ether: ethyl acetate = 6: 1), and 6.1 g of the product mesylate sugar oxime (compound 4) was obtained. The product was a yellow oil in 100% yield.
[0118] R f =0.51 (petroleum ether: ethyl acetate=4:1); 0.74 (dichloromethane: ethyl acetate=30:1).
[0119] IRv max (film): 2933, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com