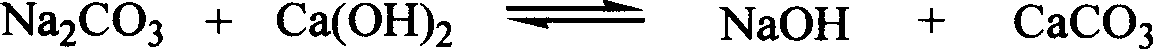New technique for preparing sodium hydroxide
A technology of soda ash and liquid caustic soda, which is applied in the direction of alkali metal compounds, alkali metal hydroxides, alkali metal oxides/hydroxides, etc., and can solve the problems of limited consumption of carbide slag, unknown market prospects, and small consumption. Achieve the effect of reducing entrainment of raw materials and products, improving mass transfer and washing effect, and improving washing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of Caustic Soda and Light Calcium Carbonate from Carbide Slag and Sodium Bicarbonate by Causticizing Reaction
[0032] Add 1.0L of water to the reaction flask, then add 500g of sodium bicarbonate, mix and stir, and heat to above 95°C, keep warm for about 0.5h to dissolve the material. Then add 480g of calcium carbide slag (wherein calcium hydroxide content is 88%) and continue to insulate and stir at 95°C. During the process, samples are taken to detect the concentration of caustic soda, and the incubating time is about 4 hours to end. While hot, it was centrifuged, purged and filtered to obtain 1040 g of caustic soda solution with a NaOH content of 20.6%, a yield of 95%, and a sodium carbonate content of 0.7%. After the filter residue was washed, it was dried and pulverized to obtain a total of 630 g of light calcium carbonate with a fineness greater than 400 mesh.
Embodiment 2
[0034] Preparation of Caustic Soda and Light Calcium Carbonate by Causticizing Reaction of Carbide Slag and Sodium Carbonate
[0035] Add 0.5L of water to the reaction flask, add 250g of sodium carbonate in portions, mix and stir, and heat to above 95°C, keep warm for about 0.5h to dissolve the material. Then add 190g of calcium carbide slag (wherein the calcium hydroxide content is 88%) in stages and continue to insulate and stir at 95°C. During the process, samples are taken to detect the concentration of caustic soda, and the incubating time is about 4 hours to end. While hot, it was centrifuged, purged and filtered to obtain 823g of caustic soda solution with a NaOH content of 21%, a yield of 95%, and a sodium carbonate content of 0.8%. After the filter residue was washed, it was dried and pulverized to obtain a total of 250 g of light calcium carbonate with a fineness greater than 400 mesh.
Embodiment 3
[0037] Using sodium chloride and ammonium bicarbonate as raw materials to prepare sodium bicarbonate first, then add quantitative calcium carbide slag to prepare caustic soda and light calcium carbonate through causticization reaction
[0038] Dissolve 360g of sodium chloride in 1000g of water at a temperature of 60°C, stir and dissolve until clear. After filtering to remove impurities, the filtrate was cooled to 35°C, 480g of ammonium bicarbonate was added, and kept stirring for 40 minutes, a large amount of precipitation appeared, the temperature was lowered to 25°C, and 500g of sodium bicarbonate solid was obtained by filtration. Add sodium chloride 350g in the filtrate, separate out the ammonium chloride crystal, after filtering, the filtrate is circulated and used to make sodium bicarbonate.
[0039] Add 1.0L of water into the reaction flask, then add 500g of sodium bicarbonate obtained in the above process, mix and stir, heat to above 95°C, and keep warm for about 0.5h t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fineness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com