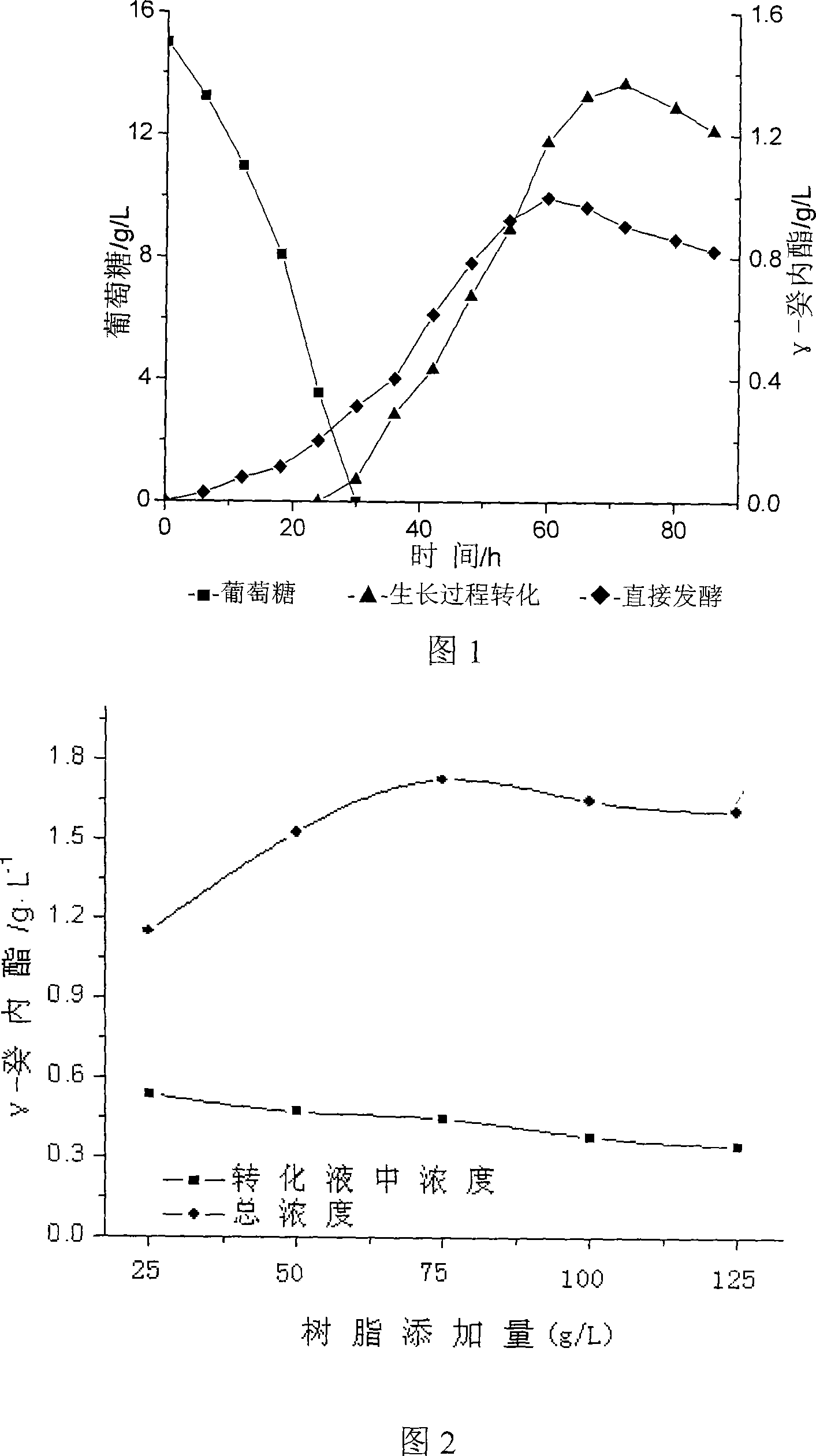Method for preparing gamma-decalactone and improving output by biotransformation and separated coupling
A technology for decanolactone and products, which is applied in the field of biological preparation of aromatic compounds, can solve the problems of complex transformation process, metabolic pathway and regulation mechanism, etc. The effect of conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 The effect of different addition methods of castor oil on the conversion of γ-decalactone.
[0026] Two different preparation methods were compared by growing cell culture method and direct fermentation with castor oil as carbon source. Growth cell transformation, that is, when the bacteria grow to the equilibrium period and the glucose is basically exhausted, castor oil is directly added to allow the microorganisms to undergo biotransformation while multiplying and growing. The growth process was used for transformation, and the yield of decanolide was significantly higher after adding castor oil for 48 hours. When castor oil is used as carbon source for direct fermentation, the product can also be prepared, but the yield is low. The result is shown in Figure 1.
Embodiment 2
[0027] Example 2 Effects of different resin additions on the conversion yield of γ-decalactone.
[0028] Add resin AB-8, the addition amount (g / L) is 25, 50, 75, 100, 125 respectively, the conversion situation of γ-decalactone is shown in Figure 2. With the increase of the amount of resin, the adsorption amount of γ-decalactone gradually increased, and the overall concentration was the highest when the amount of resin added was 75, reaching 1.73g / L. Continue to increase the amount of resin, but the overall yield did not increase significantly.
Embodiment 3
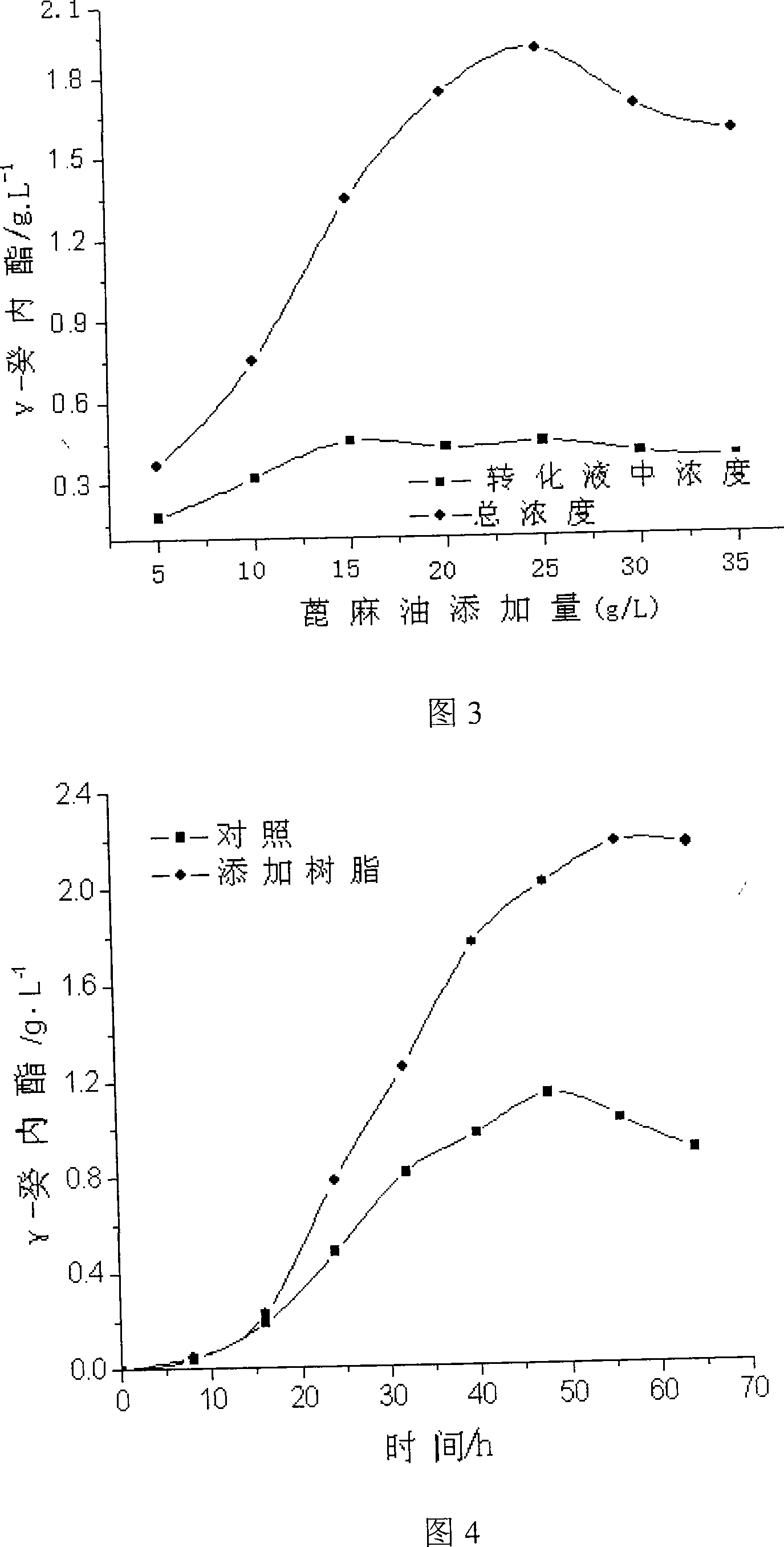
[0029] Example 3 The effect of different castor oil dosages on the conversion of γ-decalactone.
[0030] The amount of resin is constant, after conversion by adding different amounts of castor oil, the content of γ-decalactone is measured, and the results are shown in Figure 3. As can be seen from Figure 3, the decanolactone output is higher when the substrate concentration is 25g / L. There is an optimum value for the amount of substrate added, and then with the increase of substrate concentration, the yield will decrease.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com