Preparation of 1-(4-hydroxyphenyl)-2-bromopropan-1-one and application thereof
A technology of hydroxypropiophenone and bromopropane is applied in the application field of preparing ifendil and medicinal salts thereof, which can solve the problems of difficulty in post-processing, affecting later reactions, impure products, etc. Reduce the generation of side reaction impurities, the effect of suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
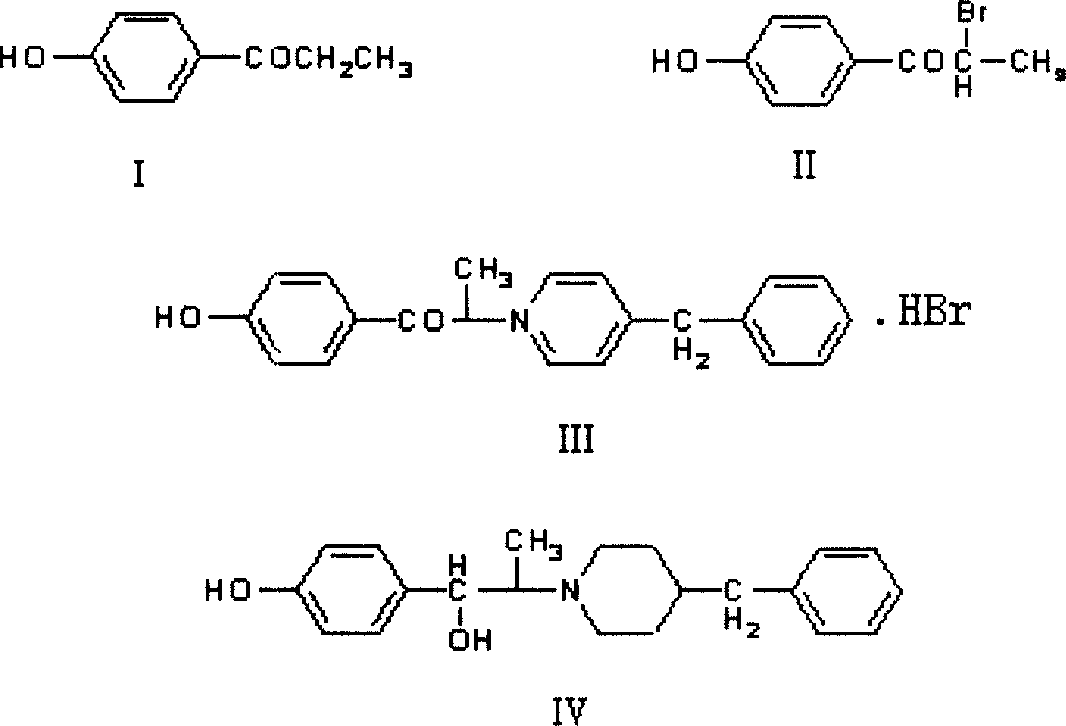
[0045] Example 1: 4.5 g (0.03 mol) of 4-hydroxypropiophenone, 14.7 g (0.066 mol) of copper bromide, 33 ml of ethyl acetate, and 22 ml of chloroform were added to the reaction flask, stirred, and heated to reflux. After 4 h of reaction, Cooling and filtration, the obtained solid cuprous bromide was washed with an appropriate amount of ethyl acetate and recovered; the washings and the filtrate were combined, 60 ml of dilute ammonia water was added, stirred, allowed to stand, separated, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, A pale yellow solution containing 1-(4-hydroxyphenyl)-2-bromopropan-1-one was obtained, which was concentrated to give a brown solid 1-(4-hydroxyphenyl)-2-bromopropan-1-one 6.8 g. HPLC: 99.0%.
Embodiment 2
[0046] Example 2: 7.5 g (0.05 mol) of 4-hydroxypropiophenone, 24.8 g (0.11 mol) of copper bromide, 50 ml of ethyl acetate and 50 ml of chloroform were added to the reaction flask respectively, stirred, and heated to reflux. After the reaction for 4 h, Cool and filter, the obtained solid cuprous bromide is washed with an appropriate amount of ethyl acetate and recovered; the washing liquid and the filtrate are combined, 100 ml of deionized water is added, stirred, left to stand, liquid separation, and the organic phase is collected and dried with anhydrous sodium sulfate and activated carbon Decolorization to obtain a pale yellow solution containing 1-(4-hydroxyphenyl)-2-bromopropan-1-one containing bromine, and concentrated to obtain a brown solid 1-(4-hydroxyphenyl)-2-bromopropane-1- Ketone 11.5g. HPLC: 98.9%.
Embodiment 3
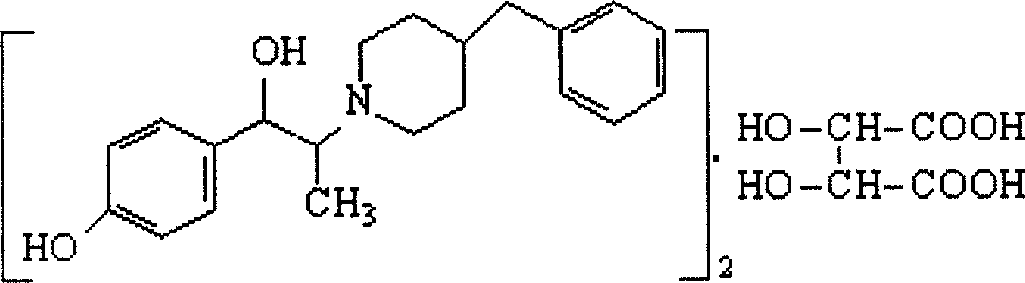
[0047] Example 3: 10.5g (0.07mol) of 4-hydroxypropiophenone, 28.1g (0.126mol) of copper bromide, 60ml of ethyl acetate and 60ml of chloroform were added to the reaction flask respectively, stirred, heated to reflux, reacted for 5h, After cooling and filtration, the obtained solid cuprous bromide was washed with an appropriate amount of ethyl acetate and recovered; the washings and the filtrate were combined, 110 ml of 2N sodium hydroxide solution was added, stirred, left to stand, separated, and the organic phase was collected and dried over anhydrous sodium sulfate. , a pale yellow solution was obtained, which was concentrated to obtain 15.2 g of 1-(4-hydroxyphenyl)-2-bromopropan-1-one as a brown solid, HPLC: 98.9%. The following operations are the same as those in Example 1, to obtain 14.1 g of Ifendil as a white solid (containing one molecule of isopropanol). Yield: 52.4%, HPLC: 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com