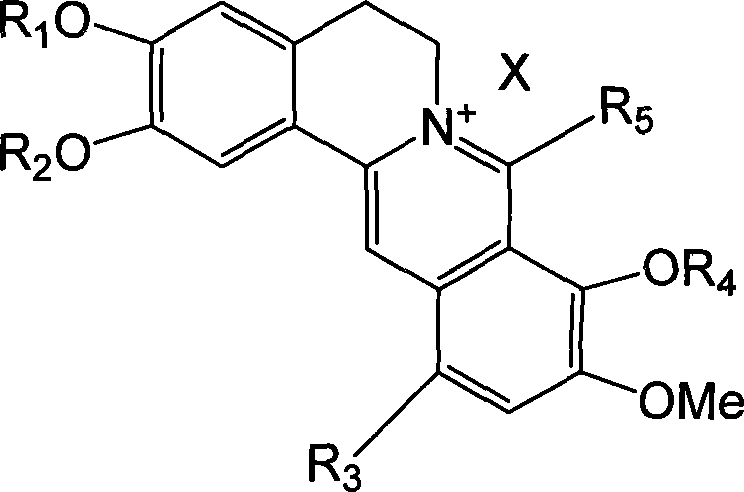
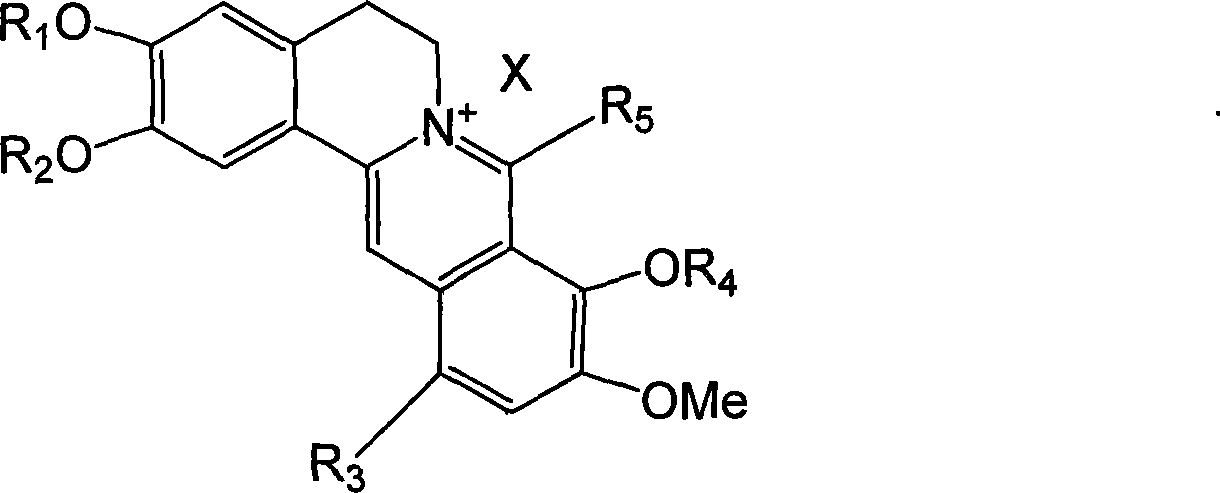
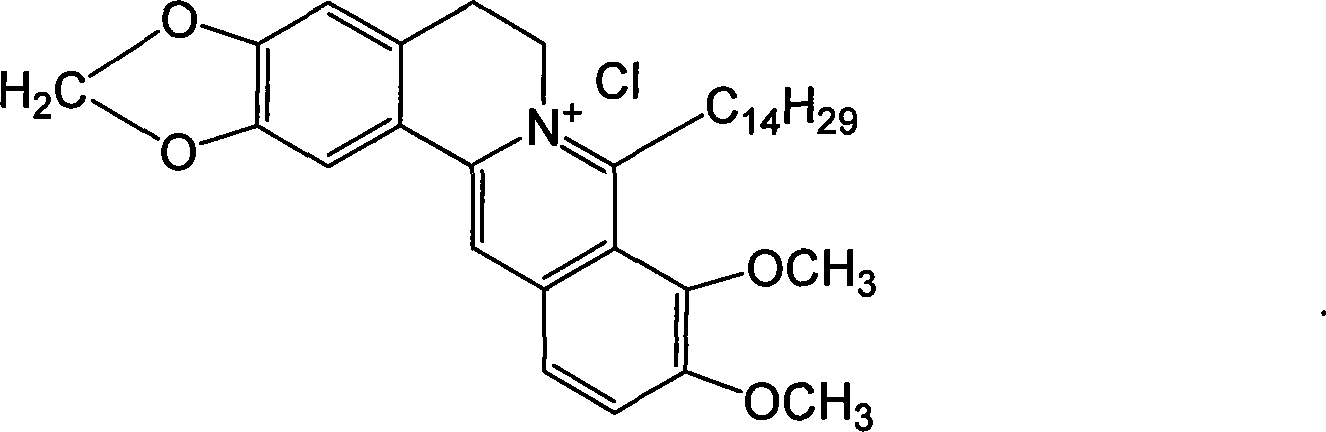
Long chain alkyl berberine salt derivative, synthetic method and use
A technology of berberine salt and long-chain alkyl group, applied in the field of new compounds and their medical uses, can solve the problems such as no literature report, no report, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: 8-tetradecylberberine hydrochloride.
[0062]
[0063] It is prepared as follows:
[0064] ①. Dry all reaction glass instruments, weigh 0.1 mol of dried magnesium chips and place in a 250 mL three-neck flask, use 40 mL of anhydrous tetrahydrofuran as the reaction solvent, add 0.1 mol of n-tetradecane chloride under nitrogen protection, and prepare corresponding Grignard reagents.
[0065] ②. Weigh 0.1mol of dry berberine hydrochloride and place it in a 500mL three-neck flask, add 100mL of anhydrous tetrahydrofuran to make a suspension of berberine hydrochloride, and then place it in an ice bath to -10°C under nitrogen protection.
[0066] ③. Slowly add the prepared Grignard reagent to the berberine suspension under nitrogen protection and ice bath, while stirring. After the addition, remove the ice bath, return to room temperature, and heat to reflux for 2 hours to complete the reaction.
[0067] ④, centrifuge the reaction solution, take the supernatant,...
Embodiment 2
[0070] Example 2: 8-octadecyl berberine bromate
[0071]
[0072] Its preparation process is as follows:
[0073] ①. Dry all reaction glass instruments, weigh 0.12mol of dried magnesium chips and place in a 250mL three-necked flask, use 100mL of anhydrous ether as the reaction solvent, add 0.06mol of brominated n-octadecane under nitrogen protection, and prepare corresponding Grignard reagents.
[0074] ②. Weigh 0.03mol of dry berberine hydrochloride and place it in a 500mL three-neck flask, add 100mL of anhydrous ether to form a suspension of berberine hydrochloride, and then ice-bath to 0°C under nitrogen protection.
[0075] ③. Slowly add the prepared Grignard reagent to the berberine suspension under nitrogen protection and ice bath, while stirring. After the addition, remove the ice bath, return to room temperature, and heat to reflux for 2 hours to complete the reaction.
[0076] ④, centrifuge the reaction solution, take the supernatant, then add diethyl ether to ex...
Embodiment 3
[0079] Example 3: 8-hexadecyl-12-bromoberberine bromate
[0080]
[0081] Its preparation process is as follows:
[0082] Dissolve 0.1 mole of 8-hexadecyl berberine bromate in 100 ml of acetic acid, add 0.5 mole of bromine, react at room temperature for 30 minutes, cool to -10°C, filter; dissolve the precipitate in ethanol and recrystallize once , to obtain 8-hexadecyl-12-bromoberberine bromate product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com