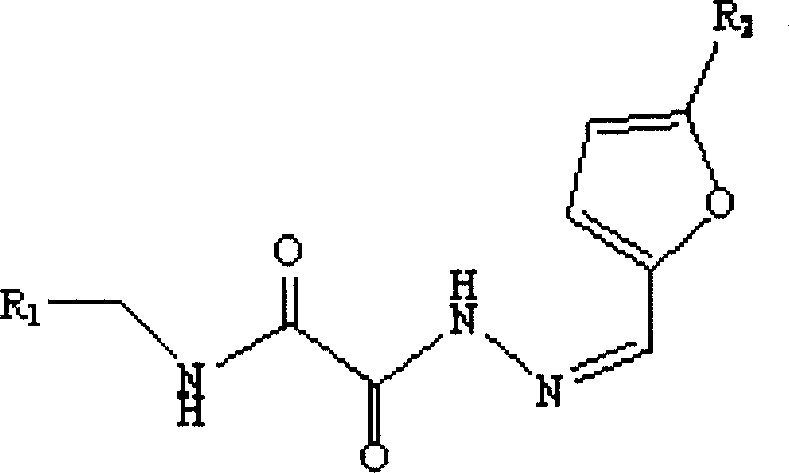
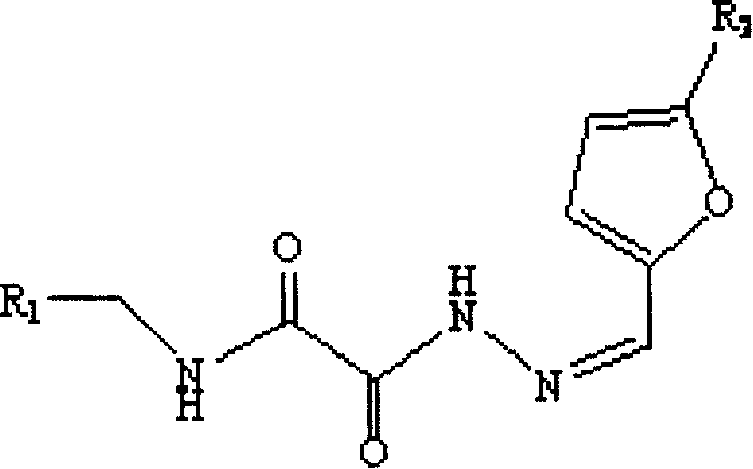
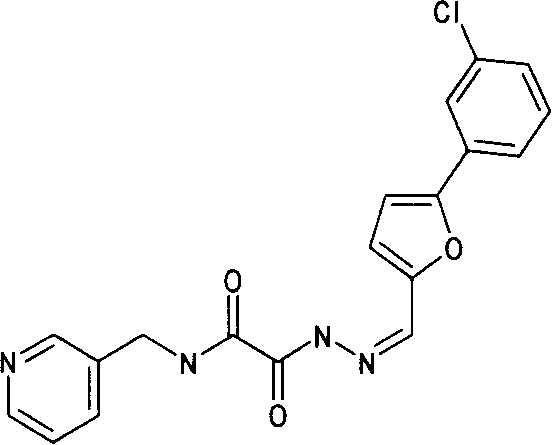
Application of cyclophilin A restrainer in preparing anti-virus medicament
An anti-AIDS and inhibitor technology, used in drug delivery, pill delivery, antiviral agents, etc., can solve problems such as anti-HIV virus CypA inhibitors that have not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Virtual Screening of CypA Small Molecule Inhibitors
[0031] The Cyclophilin A (CypA, PPIA) gene has been registered in the Human Genome Database, and its accession number is NM_021130. In the PDB protein structure database, we retrieved the X-ray diffraction crystal structure of human CypA protein (PDB code: 1CWA). This structure is the crystal structure of CypA in complex with its natural inhibitor cyclosporin A (CsA). From this structure, we determined the active site of CypA, and identified several key amino acid sites in the active site that can be inhibited by CsA. Based on these structural information, we cooperated with the Shanghai Institute of Materia Medica, Chinese Academy of Sciences to screen several small molecule databases for the active site of CypA. The small molecule databases used for screening mainly include SPECS and CNPD. Finally, ASD3 of the present invention was screened. The whole calculation process is carried out on the 64CPU-SG...
Embodiment 2
[0032] Example 2 Utilizes BIAcore Molecular Interaction Instrument to Verify Virtual Screening Results
[0033] The BIAcore molecular interaction instrument is based on surface plasmon resonance technology to track the interaction between biomolecules without any markers, thus ensuring the authenticity of the experimental results to the greatest extent. During the experiment, the target biomolecules (CypA protein) were immobilized on the surface of the sensor chip, and then the small molecule compounds were dissolved in the solvent and flowed over the surface of the chip. The monitor can track the changes in the whole process of binding and dissociation between molecules in the detection solution and target biomolecules on the chip surface in real time. Based on the binding data of BIAcore, we finally identified 12 small molecular compounds that can bind to CypA, and calculated the equilibrium-dissociation constant KD of the binding of these small molecular compounds to CypA...
Embodiment 3
[0034] Embodiment 3 compound anti-HIV-1 virus increment test
[0035] MT-2 cells were planted in a 96-well plate, 10 per well 4 indivual. The medium is RPMI1640 medium containing 10% fetal bovine serum. HIV-1 virus was used to infect the cells with 100 50% tissue infection equivalents, and at the same time, the compound was added with different concentration gradients and cultured overnight (the well without compound was used as the blank control). The next day, replace with fresh medium without small molecule compounds. On the fourth day, get 100uL of the culture supernatant, add 5% volume of Triton-X100 to obtain the virus lysate, and then use the ELISA method to measure the p24 protein content (p24 is the coat protein of HIV-1, which can be used as a measure of the number of virions) index of). Simply put, the double-antibody sandwich method is used to measure the amount of p24 protein produced in each well. Coat the plate with anti-HIV immunoglobulin overnight (pH 9.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com