Process for producing indium oxide nanocrystalline with controlled shape
A nanocrystal and indium oxide technology, which is applied in the preparation of indium oxide nanocrystals and in the field of nanomaterials, can solve problems such as easy collapse and difficult control of sample morphology, and achieve avoiding hard agglomeration, uniform morphology, and avoiding grain growth Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
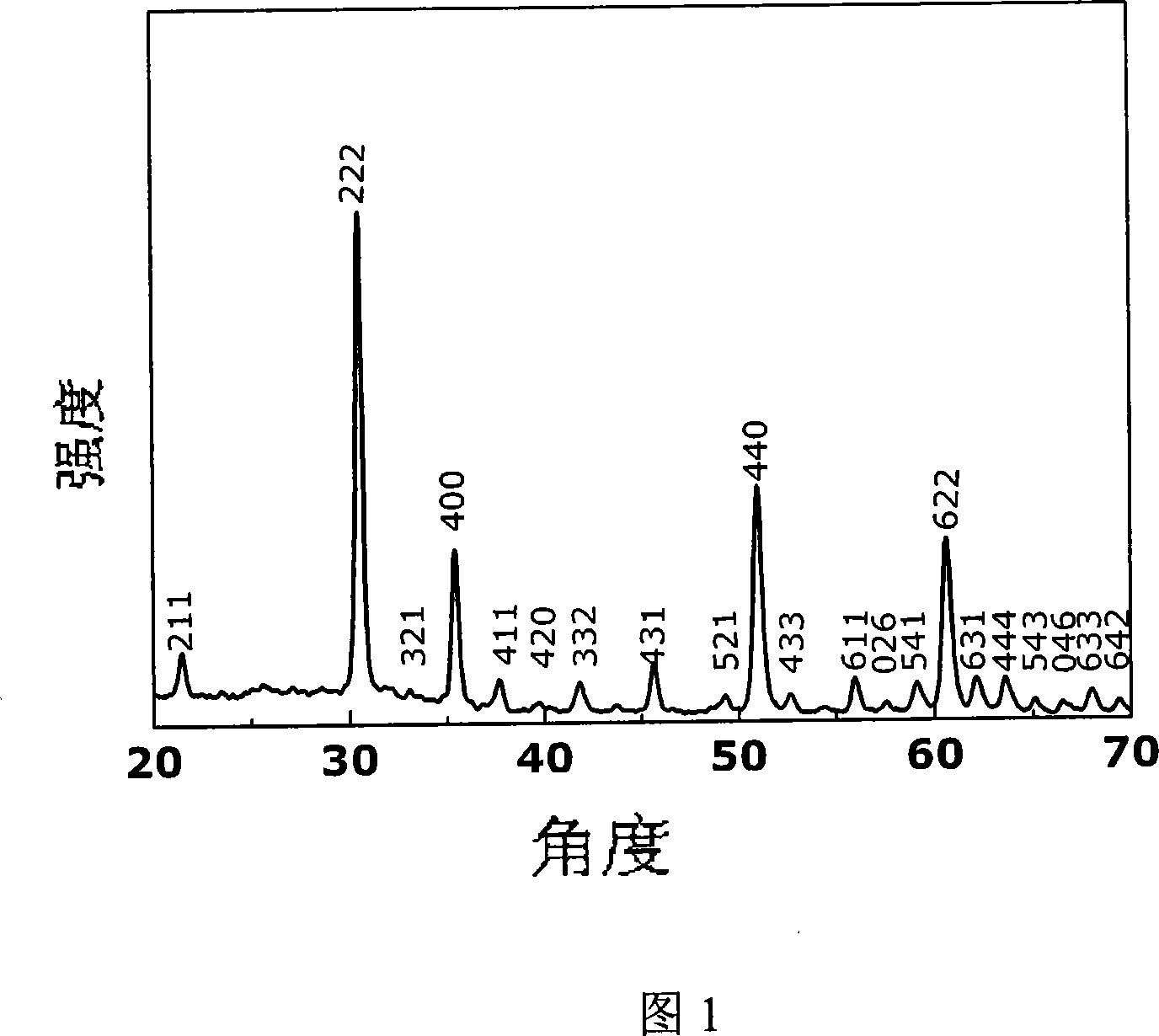
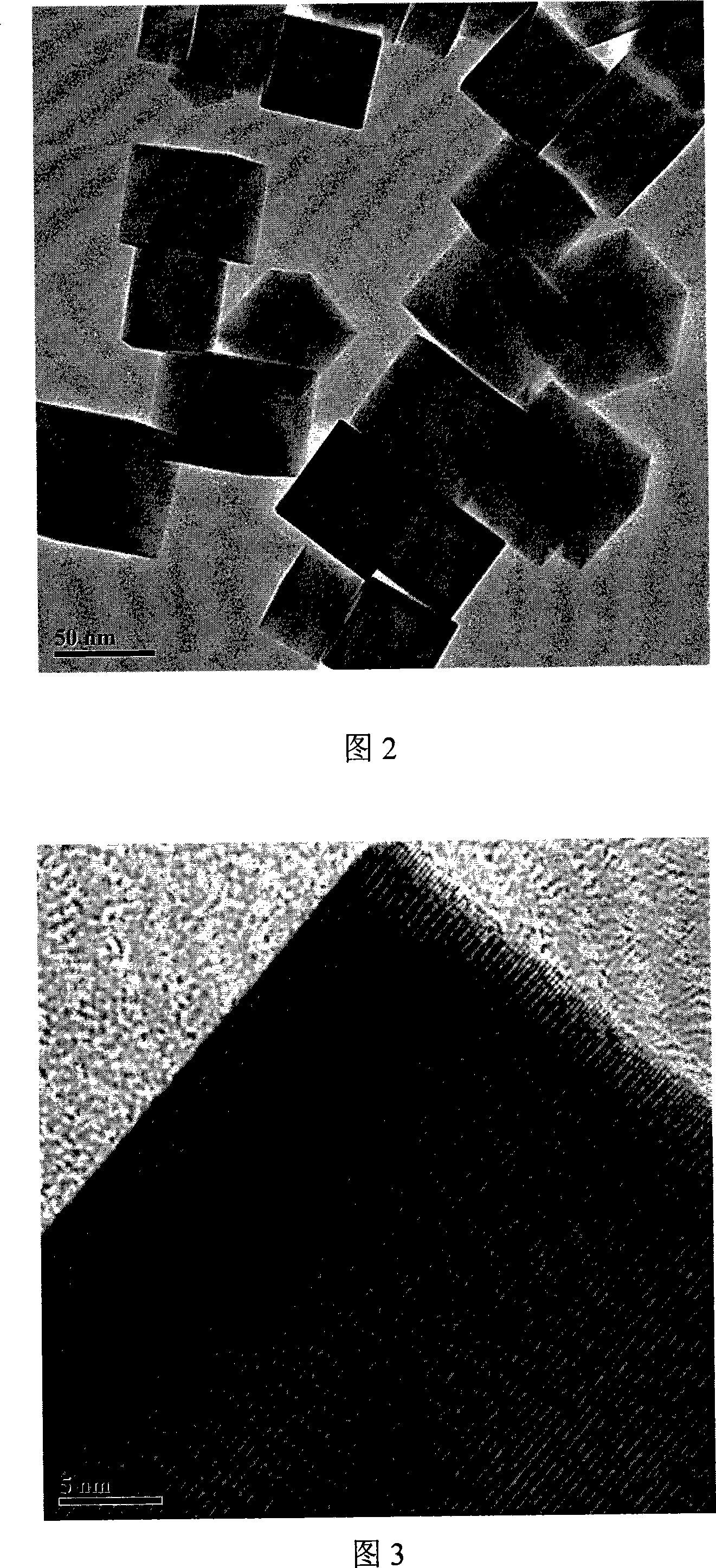
[0042] Embodiment 1, 1.6g In(NO 3 ) 3 4.5H 2 O (99.5%) was dissolved in 40ml of methanol and stirred magnetically, and 1.2g of NaOH was dissolved in another 40ml of methanol, and the latter was slowly dropped into the former solution, and a white precipitate would appear. After the titration is complete, continue to stir for 1 hour, then transfer all the obtained suspension into a hydrothermal kettle with a volume of 100ml, seal it, put it into an oven or muffle furnace and raise the temperature to 250°C at a rate of 5°C / min, and keep it warm for 10 hour, then cooled naturally, took out the reaction kettle, washed the obtained light yellow precipitate with deionized water and absolute ethanol several times, and finally filtered with suction, dried the filter cake at 80°C for 10 h, and ground to obtain the final product. (Figure 1-4)
Embodiment 2
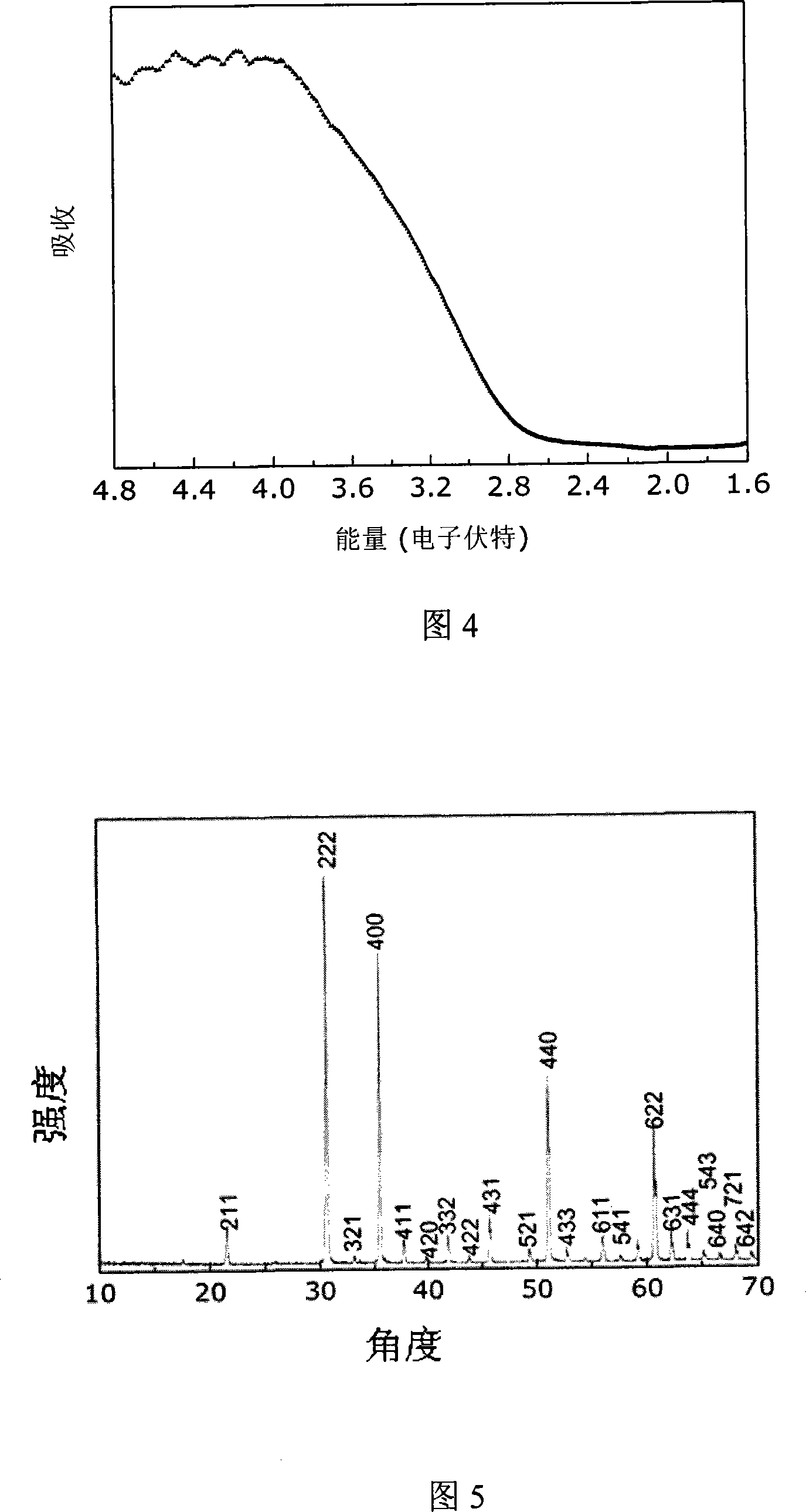
[0043] Embodiment 2, 1.6g In(NO 3 ) 3 4.5H 2 O (99.5%) was dissolved in 40ml ethanol, and magnetically stirred, and 1.2g NaOH was dissolved in another 40 ml ethanol, and the latter was slowly dropped into the former solution, and a white precipitate would appear. After the titration is complete, continue to stir for 1 hour, then transfer all the obtained suspension into a hydrothermal kettle with a volume of 100ml, seal it, put it in an oven or muffle furnace and raise the temperature to 200°C at a rate of 8°C / min, and keep it warm for 30 hour, then cooled naturally, took out the reaction kettle, washed the obtained light yellow precipitate with deionized water and absolute ethanol several times, and finally filtered with suction, dried the filter cake at 80°C for 10 h, and ground to obtain the final product. (Figure 5-6)
Embodiment 3
[0044] Embodiment 3, 0.8g In(NO 3 ) 3 4.5H 2 O (99.5%) and 0.04gFe (NO 3 ) 3 9H 2 O was dissolved in 20ml of methanol, and magnetically stirred, and another 0.6g of NaOH was dissolved in another 20ml of methanol, and the methanol solution of NaOH was slowly dropped into the former solution, and a light yellow precipitate appeared. After the titration was complete, continue to stir for 1 hour , and then transfer all the obtained suspension into a hydrothermal kettle with a volume of 100ml, seal it, put it in an oven or muffle furnace and raise the temperature to 250℃ at a rate of 5℃ / min, keep it warm for 25 hours, then cool naturally, and take out the reaction kettle , wash the obtained light blue precipitate with deionized water and absolute ethanol several times, and finally filter with suction, dry the filter cake at 80° C. for 10 h, and grind to obtain the final product. (Figure 7-9)
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com