Quality control method of asthma tablets
A quality control method and the technology of asthma tablets, which are applied to medical preparations containing active ingredients, pharmaceutical formulas, plant raw materials, etc., can solve problems such as too simple quality control indicators, inability to control product quality, and affect product quality, so as to achieve control The effect of product quality, good guidance on production, and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
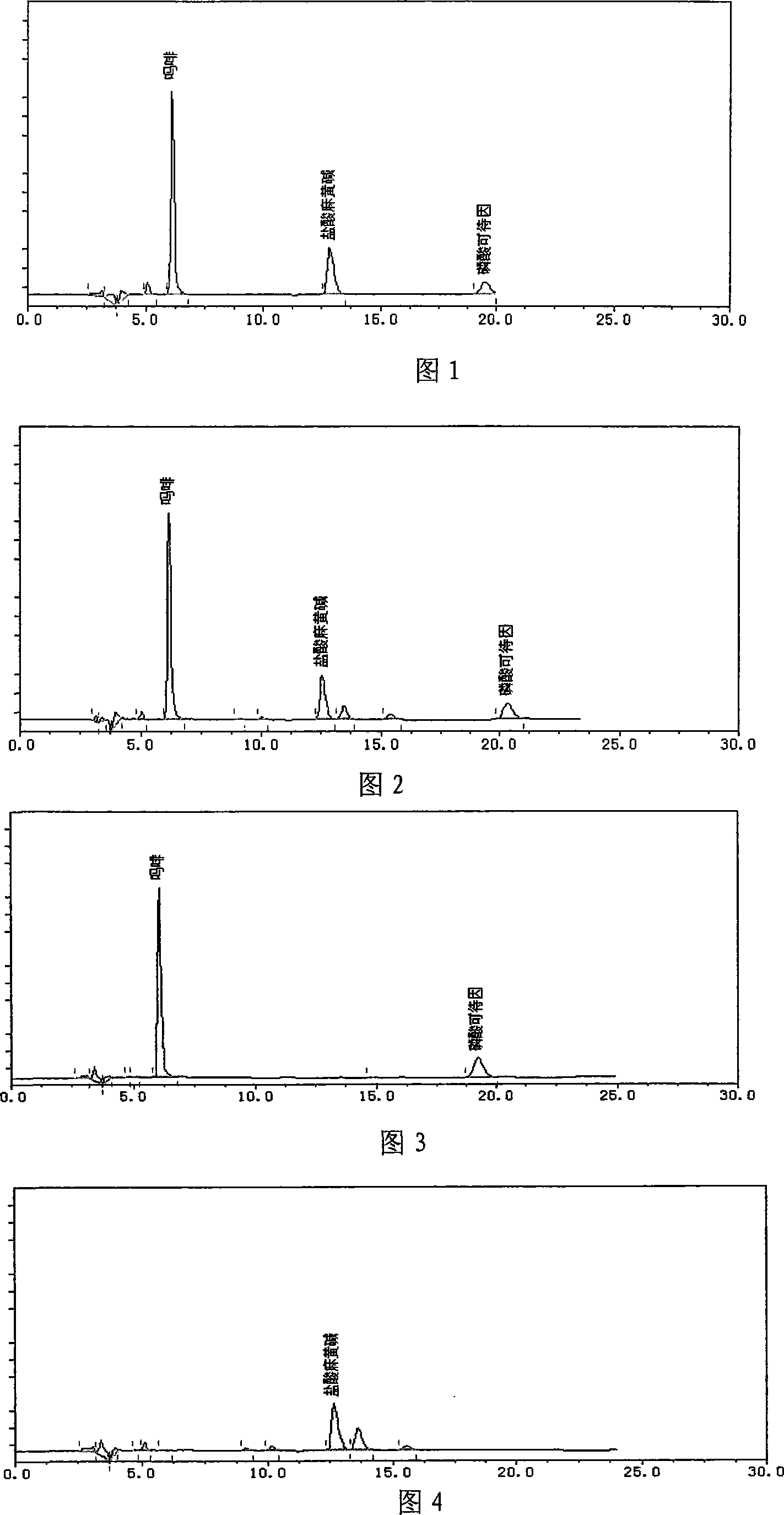
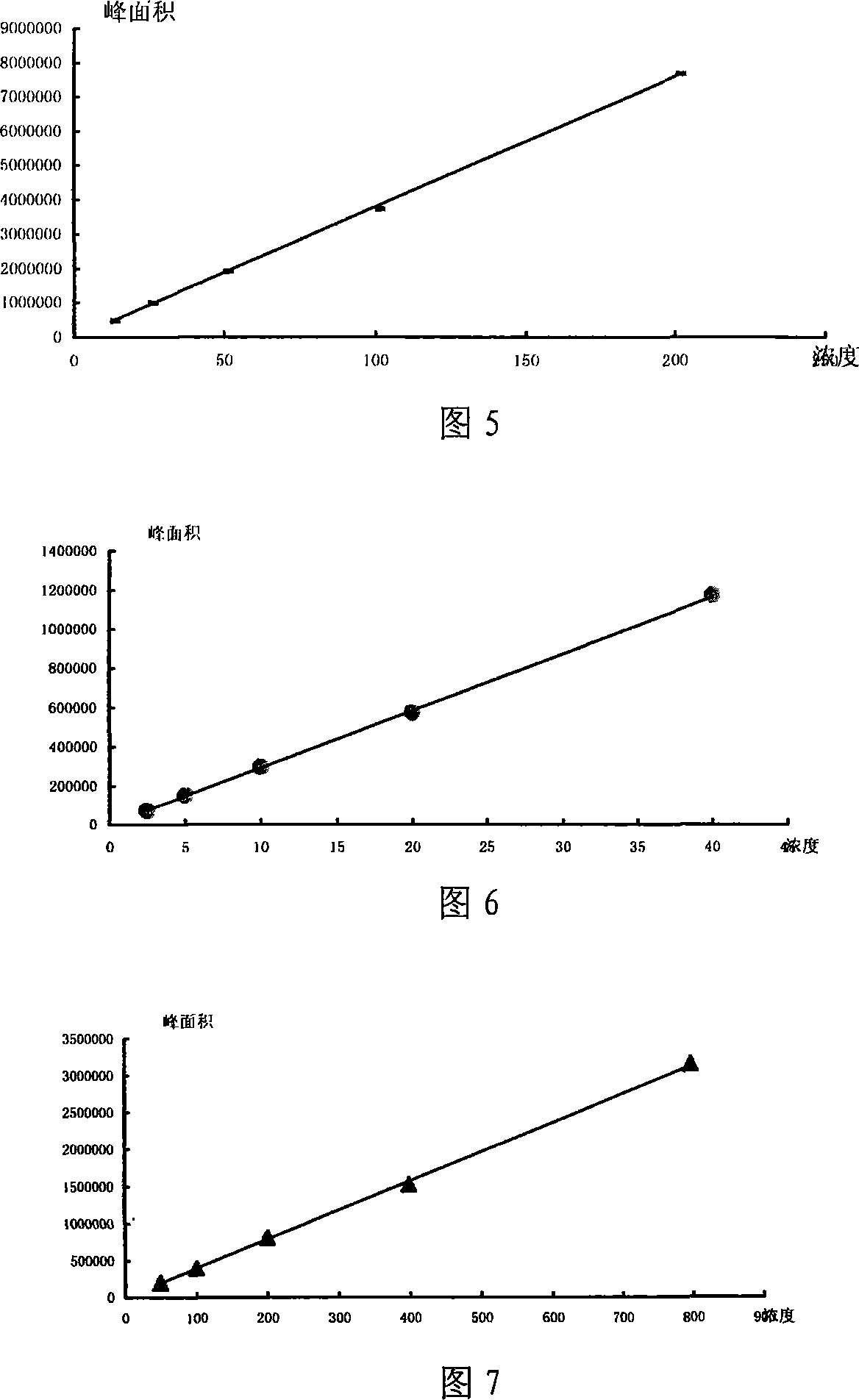
[0020] Experimental example: content determination of morphine, codeine phosphate, and ephedrine hydrochloride
[0021] (1) Selection of mobile phase:
[0022] Mobile phase 1: 0.05mol / 1 potassium dihydrogen phosphate-methanol-acetonitrile-triethylamine (80:10:10:0.3);
[0023] Mobile phase 2: 0.05mol / 1 potassium dihydrogen phosphate-methanol-acetonitrile-triethylamine (75:7:18:0.4);
[0024] Mobile phase 3: 0.05mol / 1 potassium dihydrogen phosphate-methanol-acetonitrile-triethylamine (78:7:15:0.2);
[0025] Mobile phase 4: water-acetonitrile-phosphoric acid (90:10:0.1);
[0026] Mobile phase 5: 0.01mol / 1 potassium dihydrogen phosphate-acetonitrile-phosphoric acid (90:10:0.1);
[0027] Mobile phase 6: 0.01mol / 1 potassium dihydrogen phosphate-acetonitrile-phosphoric acid-triethylamine (90:5:0.2:0.2);
[0028] Mobile phase 7: 0.01mol / 1 potassium dihydrogen phosphate-acetonitrile-triethylamine-phosphoric acid (95:5:0.4:0.2)
[0029] Through the analysis of the above comparativ...
Embodiment
[0067] Quality control methods for asthma tablets, including:
[0068] 1. Identification of Campanulaceae:
[0069] Weigh 0.5g of the fine powder of this product, put it in a stoppered test tube, add 2ml of 10% hydrochloric acid solution, bathe in water for 1h, take it out, centrifuge at 3500r / min for 10min, pour out the supernatant, adjust the pH to 9-10 with ammonia water, add 1ml of chloroform, Fully shake, 3000r / min centrifugal 10min, take chloroform layer as need testing solution; Another take 0.2g of bellflower contrast medicinal material, make contrast medicinal material solution with the same method; For the test, take 5 μl of each of the above two solutions, spot them on the same silica gel G thin-layer plate, use ethyl acetate: butanone: formic acid: water = 10:0.5:0.5:0.5 as the developer, develop, take out, and dry in the air , viewed under UV light (365nm). In the chromatogram of the test product, at the position corresponding to the chromatogram of the control ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com