Ruthenium-anthraquinone conjugates, preparation method thereof and application for optical power therapeutic photosensitizer
A technology of photodynamic therapy and conjugates, which is applied in the field of photodynamic therapy photosensitizers, ruthenium-anthraquinone conjugates and their preparation, can solve the problems of large phototoxicity and side effects, shallow treatment depth, and poor photosensitizing activity, and achieve Simple synthetic route, high inhibition rate and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
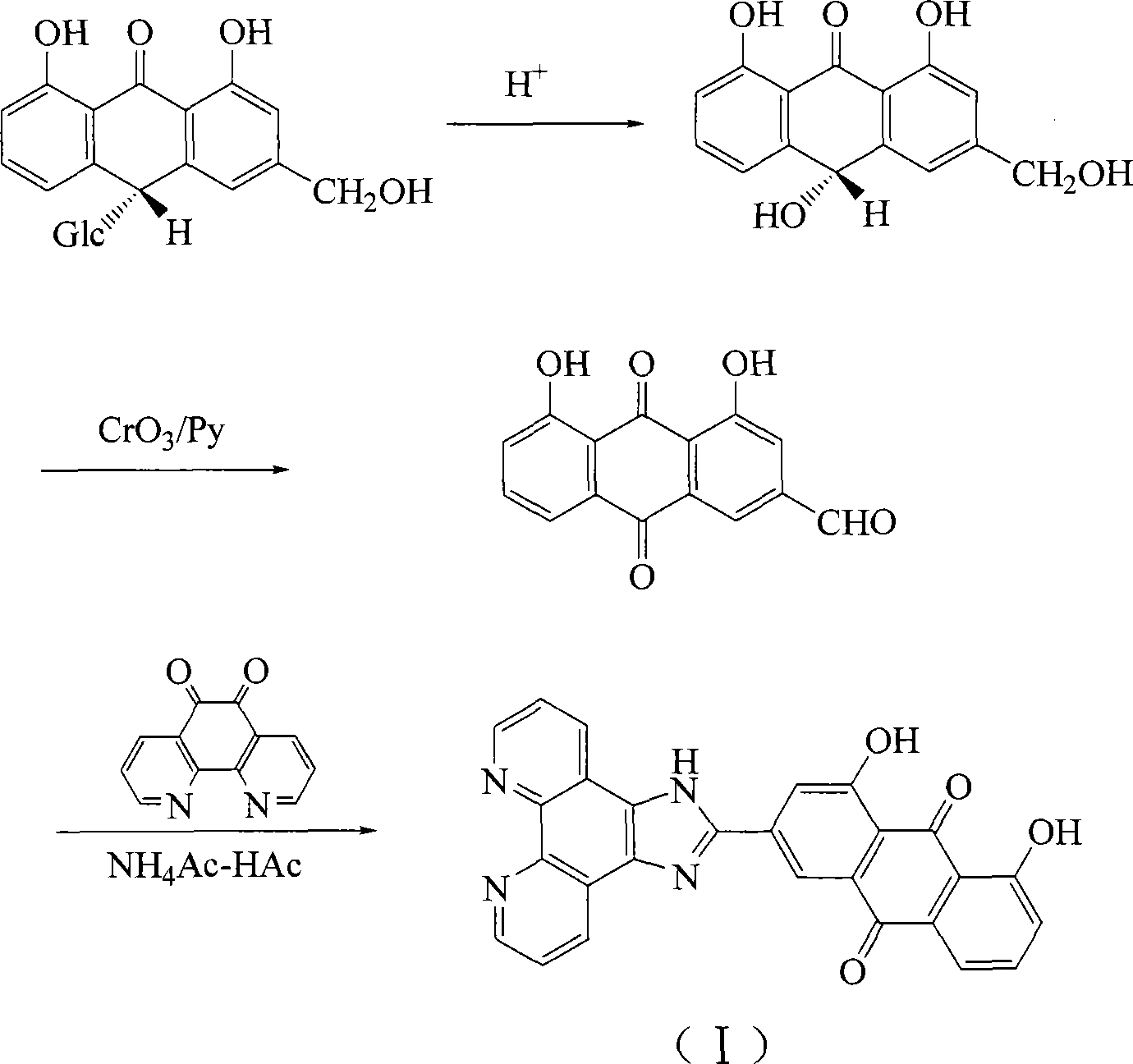
[0014] Example 1 Preparation of 1,8-dihydroxy-9,10-anthraquinone)imidazo[4,5-f][1,10]phenanthroline (HAIP):
[0015]
[0016] Add o-phenanthroline and KBr mixture into a 125ml three-necked flask, stir, and add concentrated H2 dropwise under ice-cooling 2 SO 4 and concentrated HNO 3 Composition of mixed acid (v / v, 40:20) 60mL. After the dropwise addition, continue to reflux for 3 hours, add ice water to cool, and adjust the pH value to 6-7 with NaOH. Compound with CHCl 3 Extracted 3 times, the organic phase was removed with water, Na 2 SO 4 After drying, the solvent was filtered off to obtain an orange-yellow crude product. The crude product was recrystallized from ethanol to obtain o-phenanthroline 5,6-dione.
[0017] Take 1,10-phenanthrenequinone (525mg, 2.5mmol), ammonium acetate (3.88g, 50mmol) and 1,8-dihydroxy-9,10-anthraquinone-3-aldehyde (938mg, 3.5mmol), in 10ml of ice Reflux in acetic acid for 2 hours. Add 25ml of distilled water to the deep red solution o...
Embodiment 2
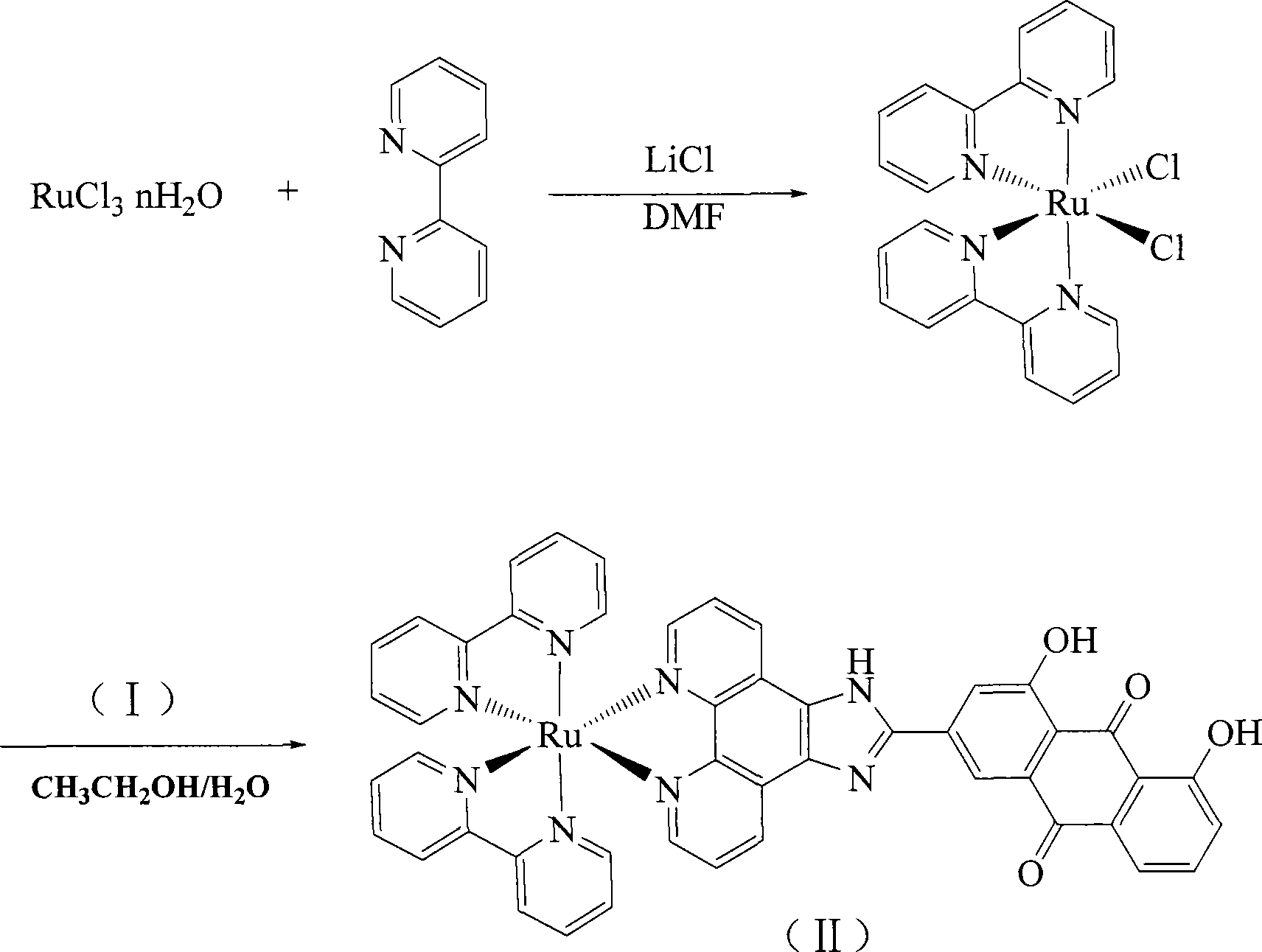
[0018] Embodiment 2 [Ru(bpy) 2 HAIP]·(PF 6 ) 2 preparation of
[0019]
[0020] cis-Ru(bpy) 2 Cl 2 2H 2 Preparation of O:
[0021] RuCl 3 ·nH 2 O (1.56g, 6mmol), bipyridine (1.87g, 12mmol), LiCl (1.68g, 28mmol), DMF (10ml) was added, and refluxed under the protection of argon for 8 hours. The reaction was cooled to room temperature and acetone (50ml) was added. The compound was left at 0°C for 24 hours to obtain purple-red crystals. The crystals were rinsed with cold water and acetone, and dried in vacuum with a yield of 81%. [Ru(bpy) 2 HAIP](PF 6 ) 2 Synthesis:
[0022] [Ru(bpy) 2 Cl 2 ]·2H 2 O (0.106g, 0.20mmol) and HAIP (0.095g, 0.20mmol), add 10cm 3 ethylene glycol. Reflux for 2 hours under argon protection. After cooling, add water (20cm 3 ), filtered to remove solid impurities. Join NH 4 PF 6 Removes dissolved impurities from the solution. The precipitated crystals were dried, dissolved in a small amount of methanol, and purified by alumina co...
Embodiment 3
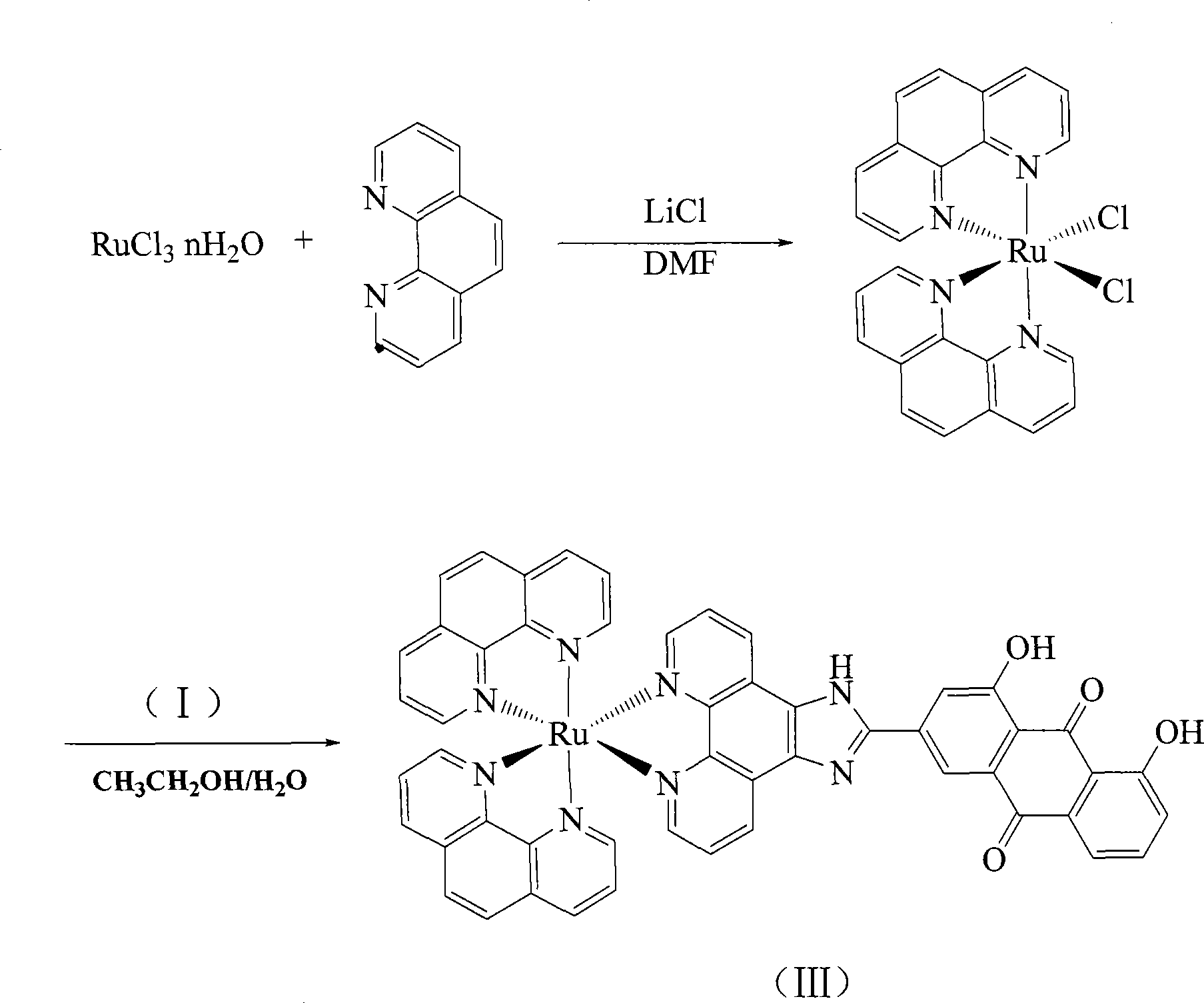
[0023] Embodiment 3 [Ru(phen) 2 HAIP]·(PF 6 ) 2 preparation of
[0024]
[0025] cis-Ru(phen) 2 Cl 2 2H 2 Synthesis of O:
[0026] RuCl 3 ·nH 2 O (1.56g, 6mmol), phenanthroline (2.16g, 12mmol), LiCl (1.68g, 28mmol), DMF (10ml) were added, and refluxed for 8 hours under the protection of argon. After the reactant was cooled to room temperature, acetone (50 ml) was added to the reactant. The reactant was left at 0°C for 24 hours to obtain purple crystals. The crystals were rinsed with cold water and acetone, and dried in vacuum with a yield of 72%. [Ru(phen) 2 HAIP](PF 6 ) 2 Synthesis:
[0027] [Ru(phen) 2 Cl 2 ]·2H 2 O (0.114g, 0.20mmol) and HAIP (0.095g, 0.20mmol) were added to 10cm 3 Ethylene glycol was refluxed for 2 hours under the protection of argon. Add water (20cm 3 ) to remove solid impurities by filtration. Join NH 4 PF 6 Removes dissolved impurities from the solution. The precipitated crystals were dried, dissolved in a small amount of meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com