Technology for preparing haw total phenolic acid part
A technology of total phenolic acid and hawthorn, which is applied in the field of medicine, can solve the problems of activity decline, etc., and achieve the effect of low cost, sufficient source of raw materials, and cheap price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Preparation of effective part
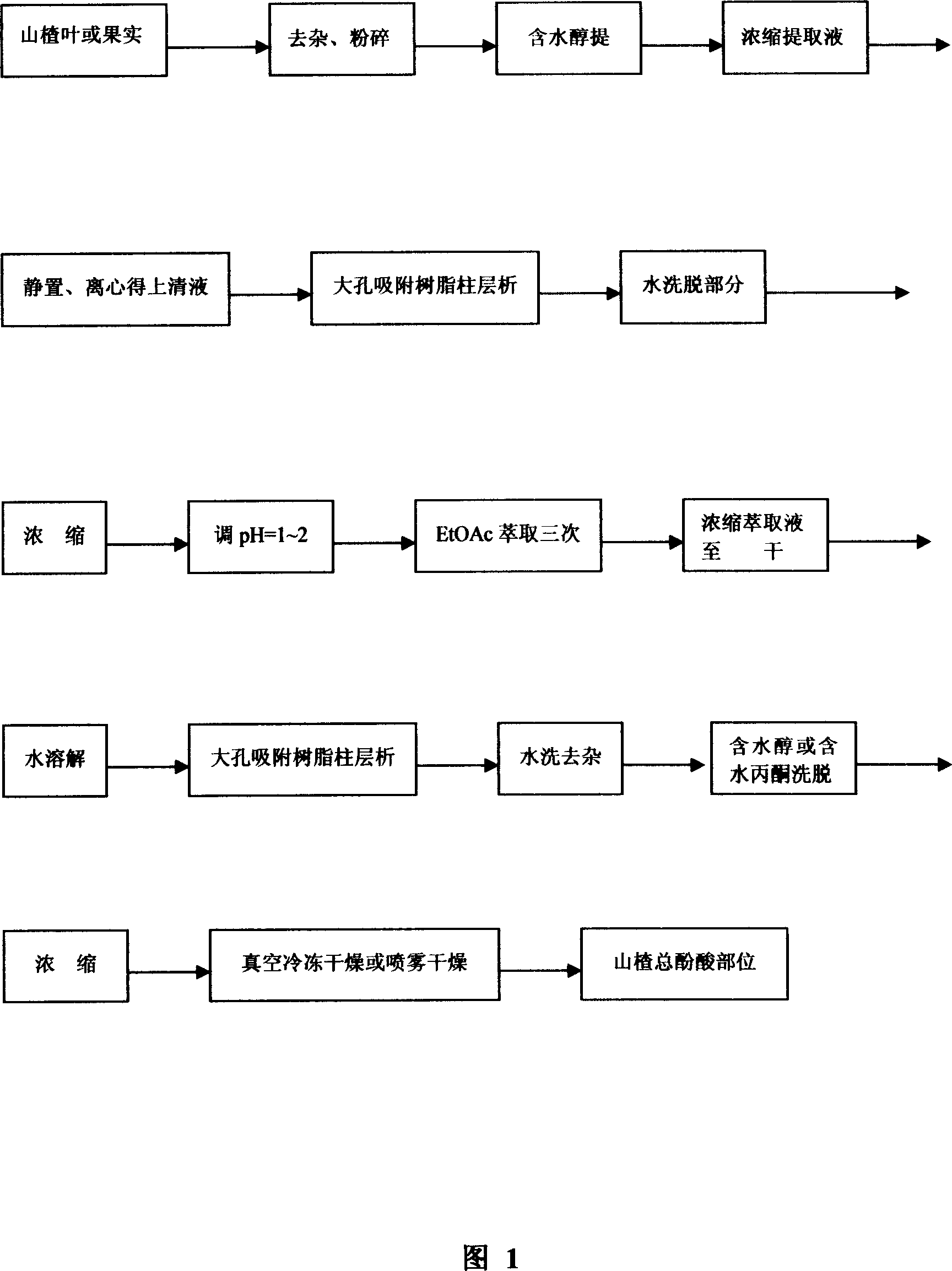
[0038] Take 100kg of dried hawthorn leaves, grind them into coarse powder, and extract with 10 times the amount of 60% ethanol under reflux for three times, combine the extracts, concentrate under reduced pressure to 50L, place for precipitation, centrifuge, and pass the supernatant through AB-8 macroporous adsorption resin Chromatography, the water eluted part adjusted the pH value to 1, extracted with ethyl acetate, the extract was concentrated under reduced pressure, dissolved in water, and then chromatographed by AB-8 macroporous adsorption resin, eluted with water until the pH value was 3 to 5, continue to elute with 60% diacetone, concentrate the eluent under reduced pressure, and spray dry to obtain 0.52 kg of light yellow powder, which is the total phenolic acid fraction of hawthorn (the yield is 0.52% based on the crude drug amount). Through assay, the purity is 84.79%.
Embodiment 2
[0039] Embodiment 2: Preparation of effective fraction
[0040] Take 100 kg of dried hawthorn fruit, crush it, and extract it by percolation with 20 times the amount of 80% methanol, combine the percolation solution, concentrate it under reduced pressure to 50 L, place it for precipitation, centrifuge, and the supernatant is subjected to D101 macroporous adsorption resin chromatography, water After adjusting the pH value of the eluted part to 2, extract it with ethyl acetate, concentrate the extract under reduced pressure and dissolve it in water, then go through AB-8 macroporous adsorption resin chromatography, and elute with water until the pH value is 3-5. Continue to elute with 20% dilute ethanol, concentrate the eluate under reduced pressure, and spray dry to obtain 0.42 kg of light yellow powder, which is the total phenolic acid fraction of hawthorn (the yield is 0.42% based on crude drug amount). Through content determination, the purity is 67.25%.
Embodiment 3
[0041] Embodiment three: the preparation of tablet
[0042] Take 100g of the part of the present invention, 80g of starch, and 5g of dextrin, mix evenly, add 10% starch slurry to make soft material, granulate with a 14-mesh nylon screen, ventilate and dry at 60-70°C, granulate with a 16-mesh sieve, add stearic acid 1.5g of magnesium and 5g of sodium carboxymethyl starch were mixed evenly, pressed into 1000 tablets, and coated. Each tablet contains 100 mg of the site of the present invention. Adults take 2 to 5 times a day, 1 to 10 tablets each time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com