Anti-HER2 single-chain antibody-cefuroxime sodium enhanced fusion protein HER2(Fv-LDM)
A technology of fusion protein and lidamycin, applied in the field of strengthening fusion protein HER2, to achieve fast clearance rate, promote tumor cell apoptosis, and good clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] . Construction and screening of HER2 phage antibody library
[0105] 1.1 Antigen protein expression, purification and mouse immunization:
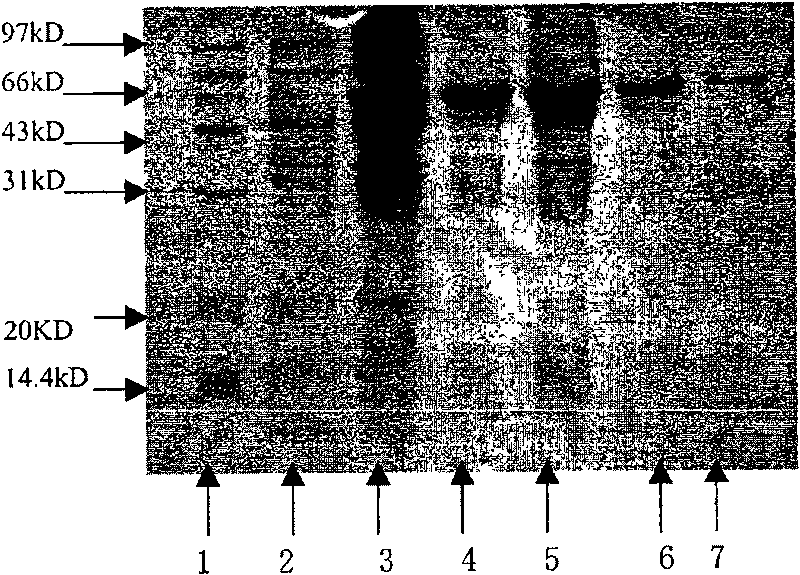
[0106] Utilize PGEX2T vector (Pharmacia product) to express HER2 extracellular domain protein in Escherichia coli BL21 (Invitrogen product), specific method refers to Han Mei et al. [Ningxia Medical Journal, 2001; 23 (3): 131-133], the optimal induction time was 4h, the induction temperature was 37°C, and the IPTG concentration was 0.8mM. The extracellular segment of GST-HER2 is expressed in the form of inclusion bodies, with a molecular weight of about 70kD, and is purified by GST-Sephrose4B column (Pharmacia product) after dialysis and renaturation ( figure 1 ). Purified HER2 extracellular segment protein 100 μg / mouse, immunized 3 female BALB / c mice of 6-8 weeks. Immunization was boosted once every two weeks thereafter. Orbital blood was collected 3 days after each booster immunization, and the purified recombinant HER2 extrac...
Embodiment 2
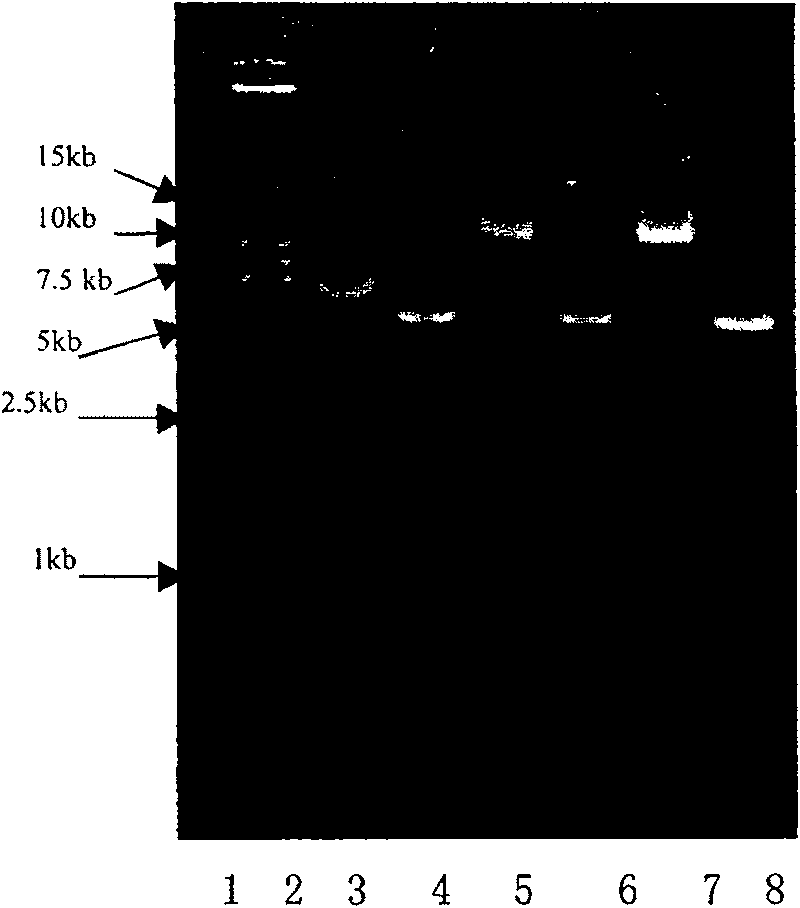
[0110] . Construction of HER2 (Fv-LDP) fusion protein recombinant expression plasmid
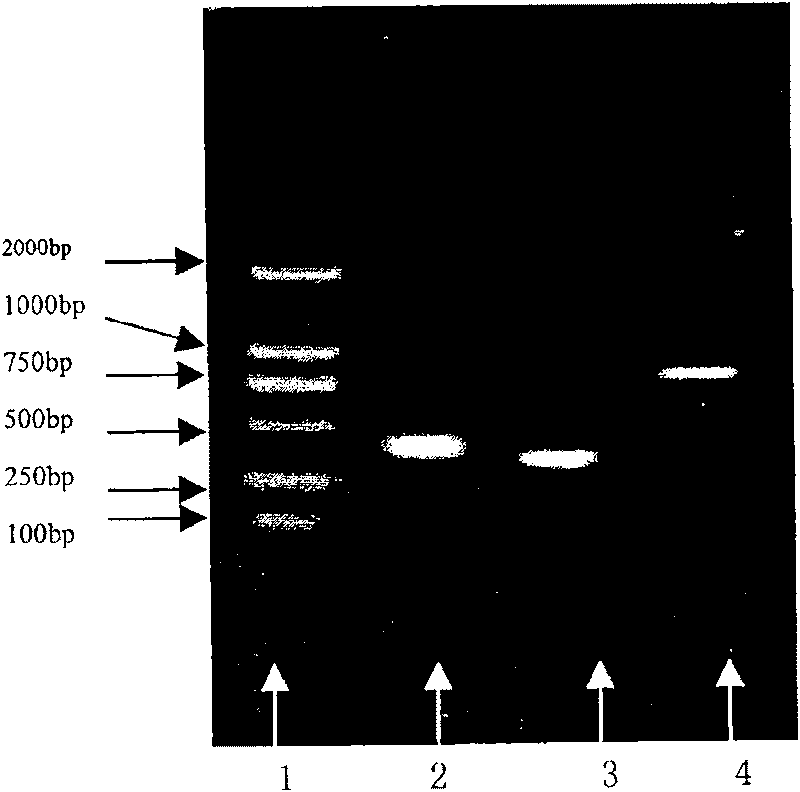
[0111] The recombinant plasmid PET-30A(+)-LDP contains the LDP gene and is preserved by our laboratory. The recombinant phagemid PCANTAB-5E contains the scFv gene, and two enzyme cutting sites, Nde I and EcoR I, are introduced by PCR. PET-30A(+)-30a Vector is a product of Novagen. PCR primers were synthesized by Invitrogen. The corresponding enzyme cutting sites were introduced respectively.
[0112] ScFv 5' end primer (PH1): 5'GATA CATATG GCCCAGGTCAAGCTGCAG 3'
[0113] Nde I
[0114] ScFv 3' Primer (PL2): 5'CG GAATTCGGATCCGCCACCGCC CCGTTTTATTTTCCAACTT 3'
[0115] EcoR I spacer
[0116]Using the recombinant phagemid PCANTAB5E-scFv as a template, PH1 as the 5' end primer, and PL2 as the 3' end primer, PCR amplification was performed to obtain a single-chain antibody gene fragment with a small peptide spacer at the C-termin...
Embodiment 3
[0117] . Fusion protein HER2 (Fv-LDP) in Escherichia coli BL21 (DE3) star TM inducible expression
[0118] The identified recombinant plasmid was transformed into Escherichia coli BL21(DE3)star TM Randomly pick recombinant transformants and inoculate them into 3ml of LB medium containing 50μg / ml kanamycin, shake and culture overnight at 37°C; inoculate at a ratio of 1:50 the next day, shake and culture at 37°C until the OD600 is 0.8, add IPTG to a final concentration of 0.8mM, induction culture for 4-6h, take an appropriate amount of bacterial liquid, and analyze the expression product localization of the whole bacteria, medium supernatant, periplasmic cavity components, soluble cytoplasmic components and inclusion bodies. The expression of exogenous protein was analyzed by 12% SDS-PAGE electrophoresis, and the fusion protein was expressed in the periplasmic cavity in a soluble form. Quantitative analysis of the gel imaging system showed that the expression level of the fusi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com