Method for preparing azoxystrobin and its analogue
A technology for pyrimidine and compound, which is applied in the field of novel preparation of agricultural fungicide azoxystrobin and its analogs, can solve problems such as deprotection and increase of raw material intermediates, and achieve the effect of simplifying production process and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
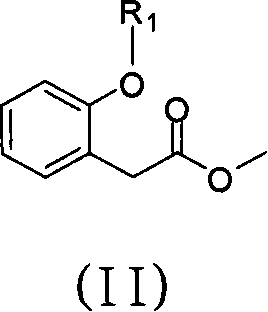
[0030] Preparation of 2-(2-(6-(2-cyanophenoxy)pyrimidine-4-oxyl)phenyl)acetic acid methyl ester (i.e. R 1 is the general formula (II) compound of 6-(2-cyanophenoxypyrimidin)-4-yl)
[0031] Under the protection of dry nitrogen, 540ml of dry N,N-dimethylformamide, 26.2g of 2-hydroxybenzonitrile, 29.8g of 4,6-dichloropyrimidine and 27.6g of anhydrous potassium carbonate were successively added to the reaction flask . Start stirring, heat to 100°C, and stir for 3 hours. Subsequently, 34.9 g of methyl 2-hydroxyphenylacetate and 13.8 g of anhydrous potassium carbonate were added, the temperature was raised to 120° C., and the reaction was stirred for 5 hours. After concentrating under reduced pressure to recover N,N-dimethylformamide, 150ml of toluene and 100ml of water were added to the residue, stirred to dissolve the solid, left to stand, and separated into layers. The organic phase was concentrated, and the obtained solid was methyl 2-(2-(6-(2-cyanophenoxy)pyrimidine-4-oxyl)p...
Embodiment 2
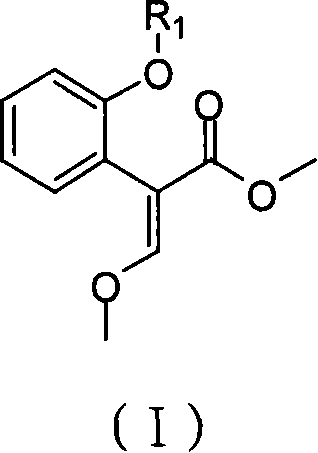
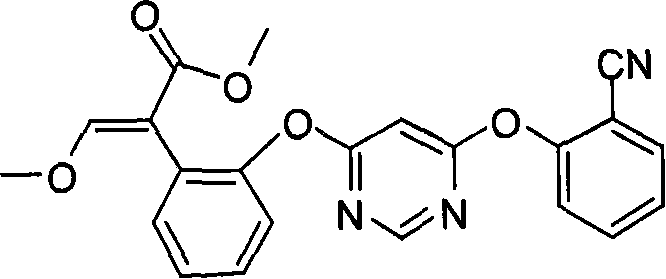
[0033] Preparation of (E)-2-{2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3-methoxymethyl acrylate (Azoxystrobin)
[0034]
[0035] Under the protection of dry nitrogen, 150ml of dichloromethane and 11.4g of titanium tetrachloride were successively added into the reaction flask, and then 6.4g of trimethyl orthoformate was added under vigorous stirring. After stirring at room temperature for 1 hour, the reaction liquid was cooled to 0°C, and 18.1 g of methyl 2-(2-(6-(2-cyanophenoxy)pyrimidine-4-oxyl)phenyl)acetate was added. After stirring for 30 minutes, 12.1 g of triethylamine was added and stirred at room temperature for 1 hour. The resulting reaction mixture was washed with 40 ml of 10% hydrochloric acid, dried over anhydrous magnesium sulfate, and stripped in vacuo to obtain a brown viscous substance. The brown sticky substance was dissolved in 100ml of hot toluene, cooled to room temperature, and 0.9g of benzyltriethylammonium chloride, 15g of 20% aqueous sodium hyd...
Embodiment 3
[0037] Preparation of (E)-2-(2-(6-chloropyrimidine-4-oxyl)phenyl)-3-methoxymethyl acrylate
[0038]
[0039]Under the protection of dry nitrogen, 150ml of dichloromethane and 11.4g of titanium tetrachloride were successively added into the reaction flask, and then 6.4g of trimethyl orthoformate was added under vigorous stirring. After stirring at room temperature for 1 hour, the reaction solution was cooled to 0° C., and 13.9 g of methyl 2-(2-(6-chloropyrimidine-4-oxy)phenyl)acetate was added. After stirring for 30 minutes, 12.1 g of triethylamine was added and stirred at room temperature for 1 hour. The resulting reaction mixture was washed with 40 ml of 10% hydrochloric acid, dried over anhydrous magnesium sulfate, and stripped in vacuo to obtain a brown viscous substance. The brown sticky substance was dissolved in 100ml of hot toluene, cooled to room temperature, and 0.9g of benzyltriethylammonium chloride, 15g of 20% aqueous sodium hydroxide solution and 12.6g of dime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com