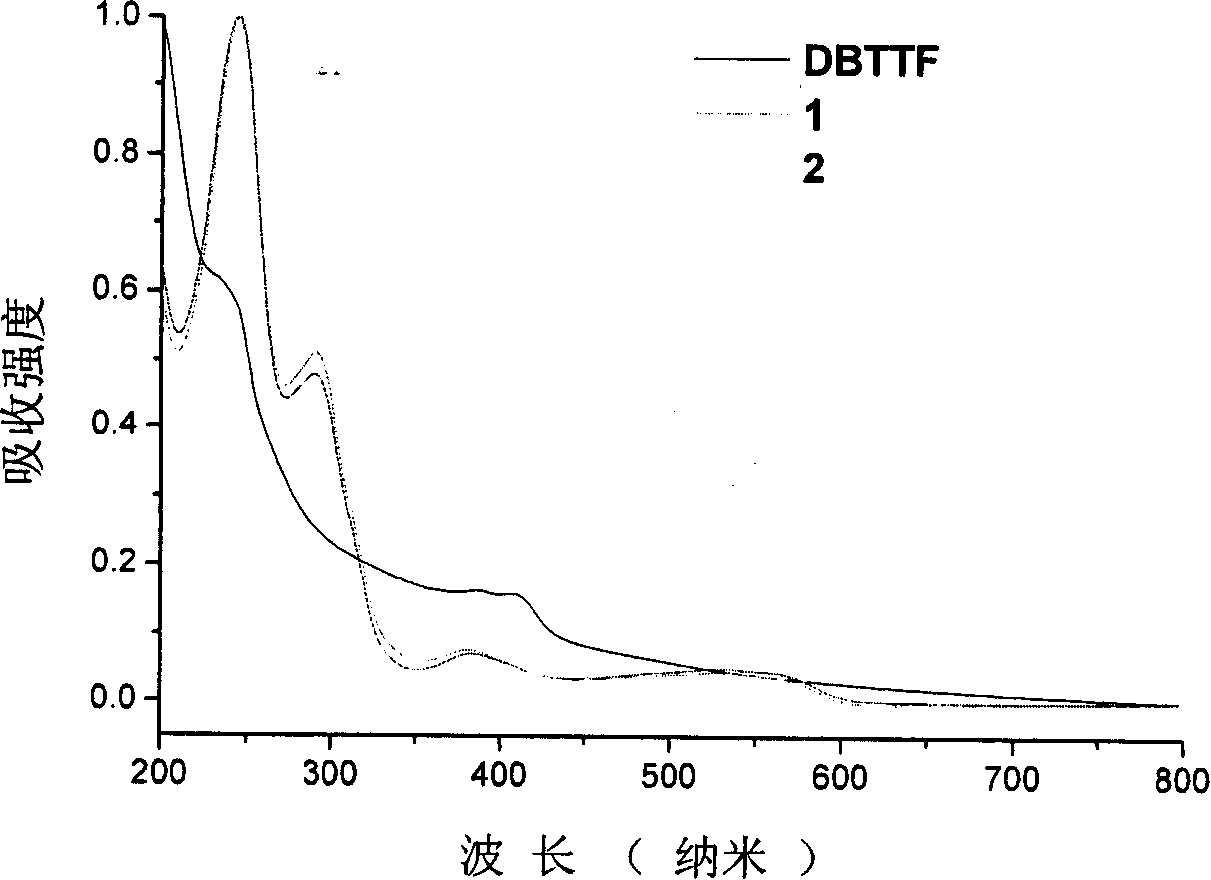
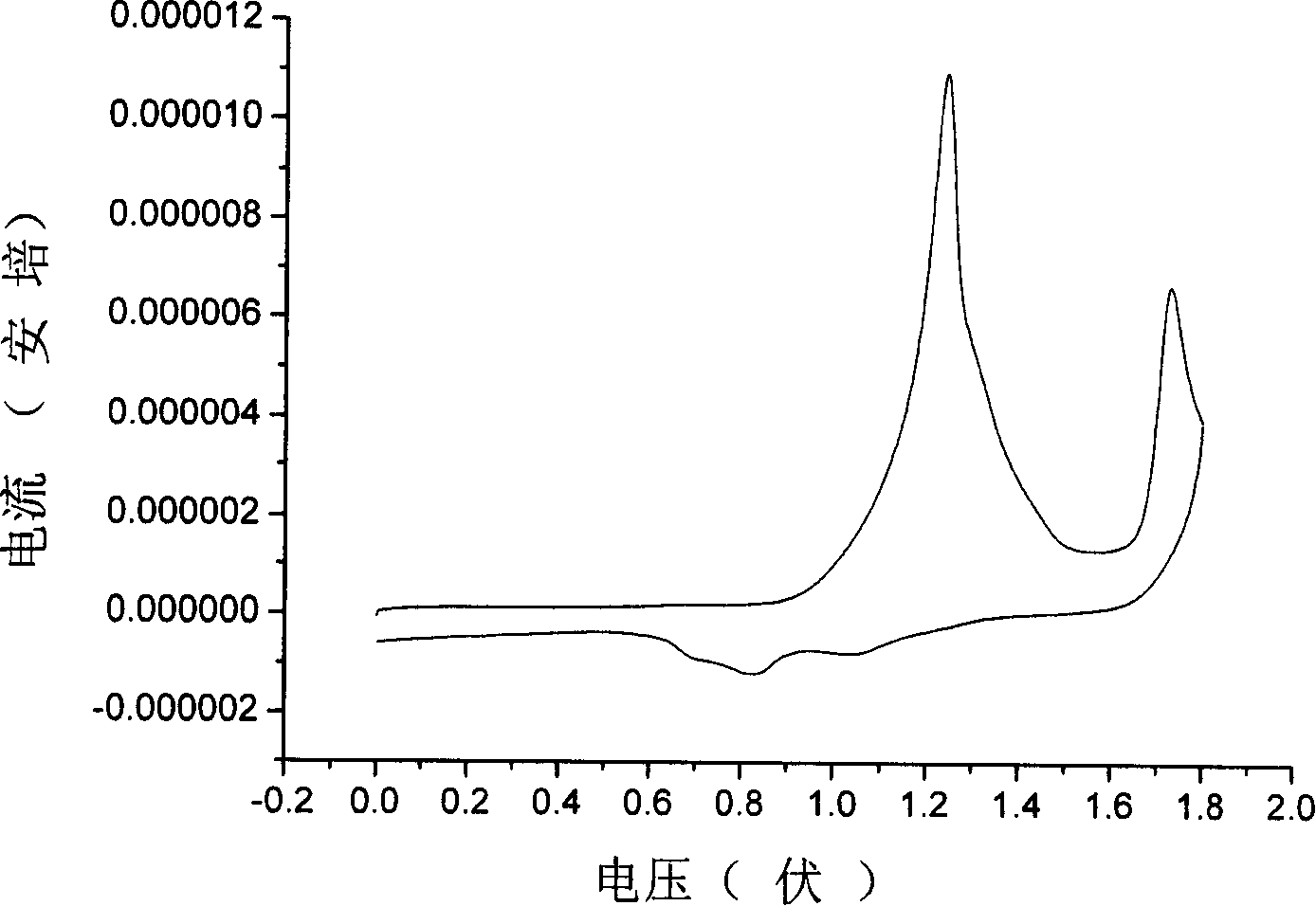
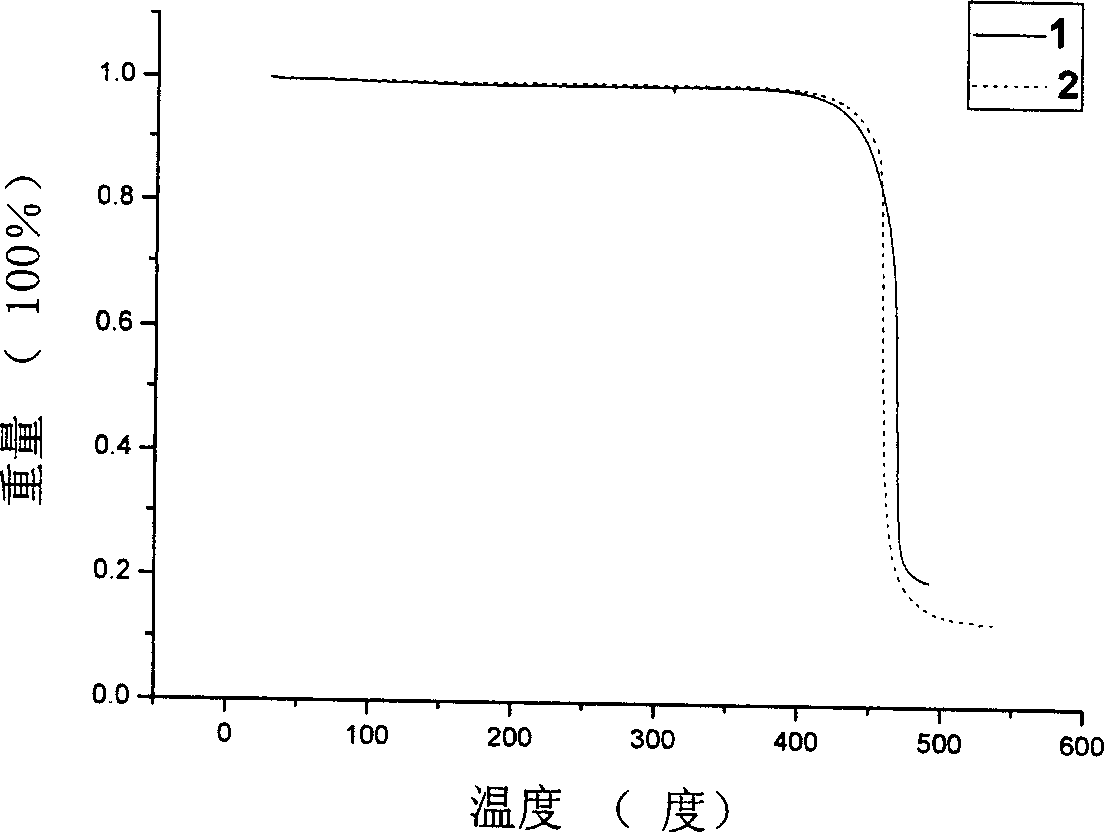
Dibenzo tetrathiafulvalene tetracarbonyl imide derivative and preparation method and uses thereof
A technology of dibenzotetrathiafulvalene tetracarbonyl diimide and benzotetrathiafulvalene tetracarbonyl diimide, which is applied in the field of organic semiconductor materials and can solve the problem of poor compound derivation ability and TTF derivatives Lack of synthetic routes, difficult synthesis and other problems, to achieve the effects of low synthesis cost, good application prospects, and high mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1, N, N'-dibutyl-dibenzotetrathiafulvalene-2,3,6,7-tetracarbonyl imide (compound 1)
[0038] Under the protection of nitrogen, 6.0g (28mmol) of 4,5-dichlorophthalic anhydride, 4.1g (56mmol) of n-butylamine, and 100mL of propionic acid were added to a 250ml three-neck flask, and the mixture was refluxed at 140°C for 3 hours. The reaction solution was cooled to room temperature, the solvent was rotated off under reduced pressure, and recrystallized in methanol to obtain 6.5 g of a white crystalline solid (Compound 3), with a yield of 85.5%.
[0039] Mass spectrum: [MS (EI)] m / z: 271 (M + ).
[0040] Elemental analysis: Molecular formula: C 12 h 11 Cl 2 NO 2 ;Theoretical value: C, 52.96H; 4.07; N, 5.15;
[0041] Found: C, 52.97; H, 4.13; N, 5.13.
[0042] H NMR spectrum: 1 H-NMR (300MHz, CDCl 3)δ (ppm): 0.92-0.97 (t, J = 7.30Hz, 3H), 1.31-1.39 (m, 2H), 1.65 (m, 2H), 3.65-3.70 (t, J = 7.23Hz, 2H), 7.91(s, 2H).
[0043] NMR carbon spectrum: 13 C-NMR (75MH...
Embodiment 2
[0061] Example 2, N, N'-dihexyl-dibenzotetrathiafulvalene-2,3,6,7-tetracarbonyl imide (compound 2)
[0062] Compound 4 is the same as the synthesis of compound 3 in Example 1, using n-hexylamine as the amine (octylamine or n-dodecylamine, etc. can also be used): the yield is 86.5%.
[0063] Mass Spectrum: [MS(EI)] m / z: 299 (M + ).
[0064] Elemental analysis: Molecular formula: C 14 h 15 Cl 2 NO 2 ;Theoretical value: C, 56.02; H, 5.04; N, 4.67;
[0065] Found: C, 56.04; H, 5.08; N, 4.63.
[0066] H NMR spectrum: 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 0.85-0.89 (t, J = 6.41Hz, 3H), 1.30 (s, 6H), 1.65 (m, 2H), 3.64-3.68 (t, J = 7.26Hz, 2H), 7.90 ( s, 2H).
[0067] NMR carbon spectrum: 3 C-NMR (75MHz, CDCl 3 )δ (ppm): 12.8, 21.3, 25.3, 27.3, 30.2, 37.4, 124.1, 130.2, 137.6, 165.2 (CO).
[0068] Synthesis of compound 6 with compound 5: the yield is 61%.
[0069] Mass spectrum: [MS(EI)] m / z: 475 (M + ).
[0070] Elemental analysis: Molecular formula: C 28 h 29 NO 2 S ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical bandgap | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com