Recombinant polypeptide having islets beta- cell protection function
A technology for recombinant polypeptides and protective effects, applied in the field of biomedicine, can solve problems such as cheap, laborious, and time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Cloning of embodiment 1 recombinant cSHAP gene
[0031] (1) Extraction of total RNA from shark (Chiloscyllium Plagiosum) liver
[0032] (2) Wu Wutong's laboratory obtained the N-terminal sequence LVGPIGAVGP of the natural shark liver active peptide in the previous work, and designed a merger primer: 5'-AA(C)TIGTIGGGICCIATC(T)GGIGCIG-3' as the upstream primer, and the downstream primer: oligodT18;
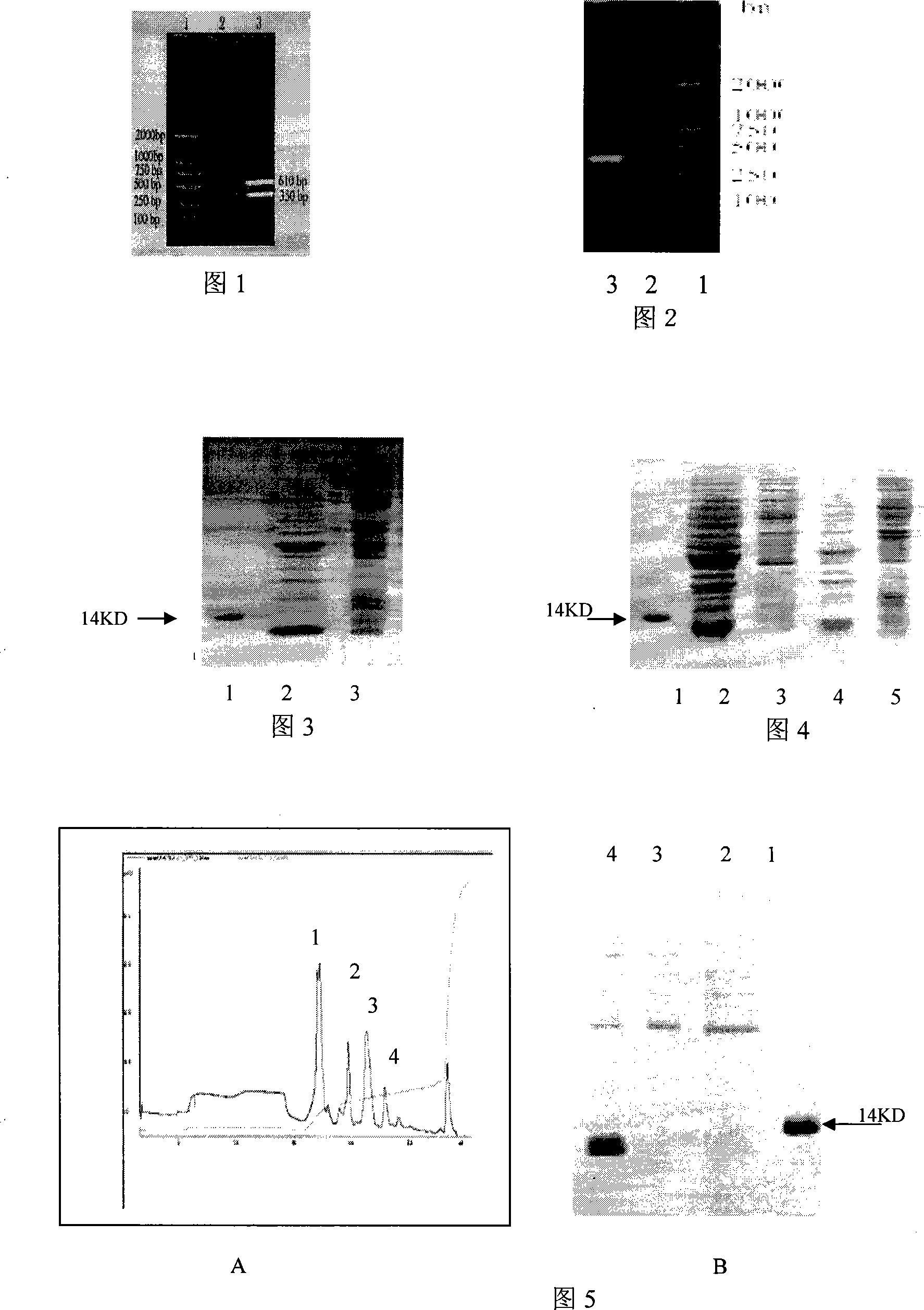
[0033] (3) Using shark liver cDNA as template, by PCR method (94 DEG C for 45s, 56 DEG C for 50s, 72 DEG C for 1min, 30 cycles; 72 DEG C for 10 min to amplify the SHAP coding sequence (accompanying drawing 1); obtain two gene fragments , the sizes are 350bp and 610bp respectively, the molecular weight of the 350bp gene partial code protein is similar to the natural active protein, so we choose the 350bp gene sequence as the coding sequence of the recombinant protein;
[0034] (4) The PCR product is cloned into the vector pGEM-T-easy to obtain the vector pT-SHAP containing th...
Embodiment 2
[0042] Example 2 Establishment of cSHAP secretion expression, separation and purification process
[0043] (1) Screening of cSHAP secretion and expression conditions
[0044] The cloned bacteria were transferred into LB medium, and the expression was induced at different culture temperatures (37°C, 28°C) with different inducer IPTG concentrations (1mM, 0.5mM, 0.1mM); Proteoproteins, as well as cytoplasmic proteins, were analyzed by 15% SDS-PAGE for protein expression; SHAF was secreted and expressed at 0.5mM and cultured at 28°C (accompanying drawing 3);
[0045] (2) Separation and purification of cSHAP
[0046] A. Extraction of periplasmic protein: Induced expression bacterial solution, centrifuged at 4°C, 12000r / min for 5 minutes, collected bacterial cells; suspended bacterial cells in ice-cold sucrose buffer solution with 1 / 10 volume of original bacterial liquid, stirred slowly in ice bath 15min. Then centrifuge at 12000r / min at 4°C for 5min to collect the bacteria; susp...
Embodiment 3
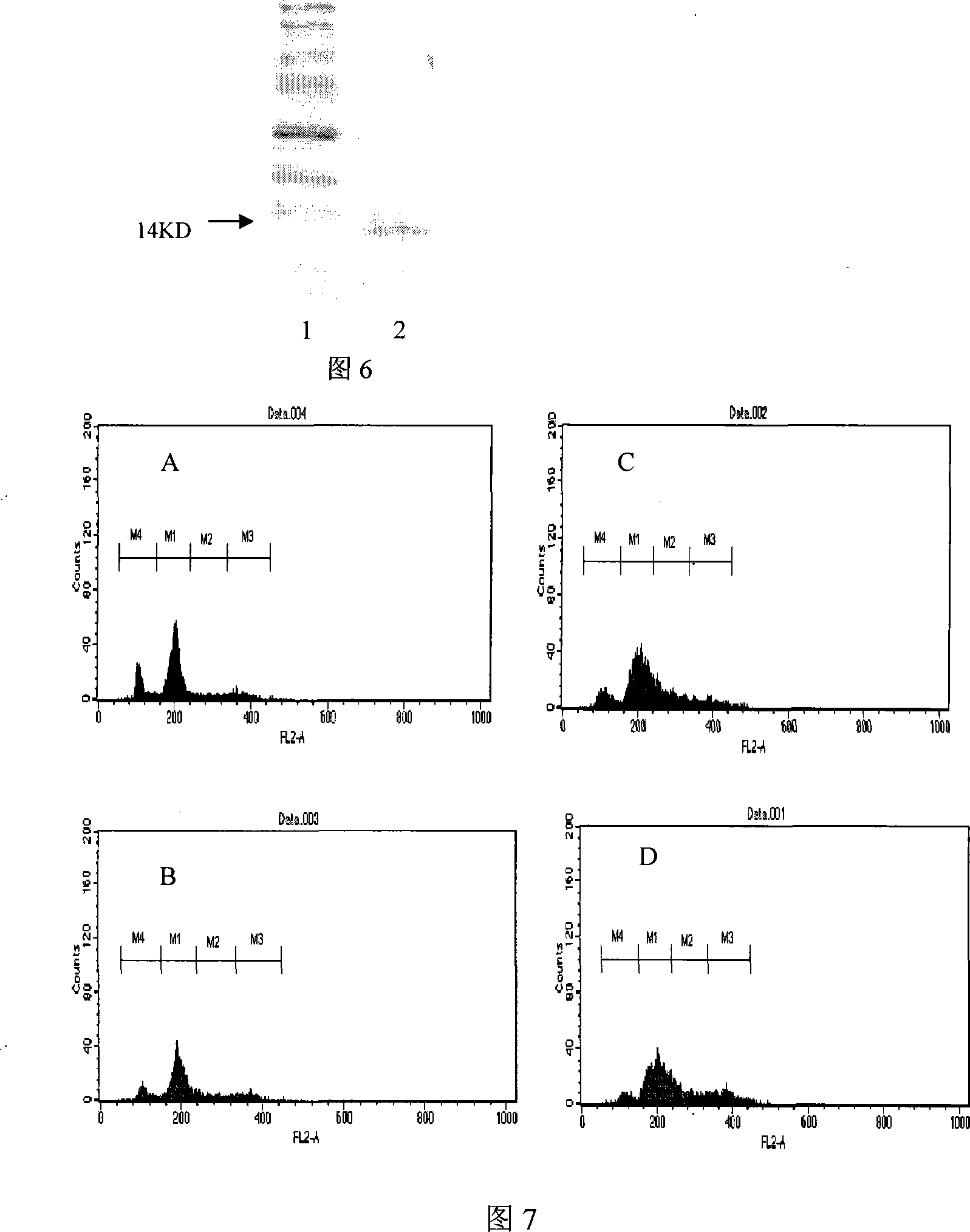
[0051] The mouse insulinoma β-cell strain NIT-1 was used as the target cell, and streptozotocin (STZ) had a damaging effect on the target cell. Take the NIT-1 cells in the logarithmic growth phase, digest them with trypsin, and use 5×10 4 Inoculate 6-well plates at a concentration of 3ml per well, culture for 24 hours, and add STZ solution to each test well at a final concentration of 5mM / L after the cells have adhered to the wall. After 1 hour, add the final concentration to each well of the test group at the same time 45 μg / ml, 15 μg / ml, 0 μg / m1 of the recombinant protein cSHAP dissolved in DMEM high-glucose medium (containing 0.5% fetal bovine serum), cultured at 37°C for 24 hours in a 5% CO2 incubator, digested and collected each Group cells, wash cells with pre-cooled PBS, fix cells with ice-cold 70% ethanol overnight, collect cells, suspend cells in each group with pre-cooled PBS, add RNaseA, act at 37°C for half an hour, add stain PI and place at room temperature for 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com