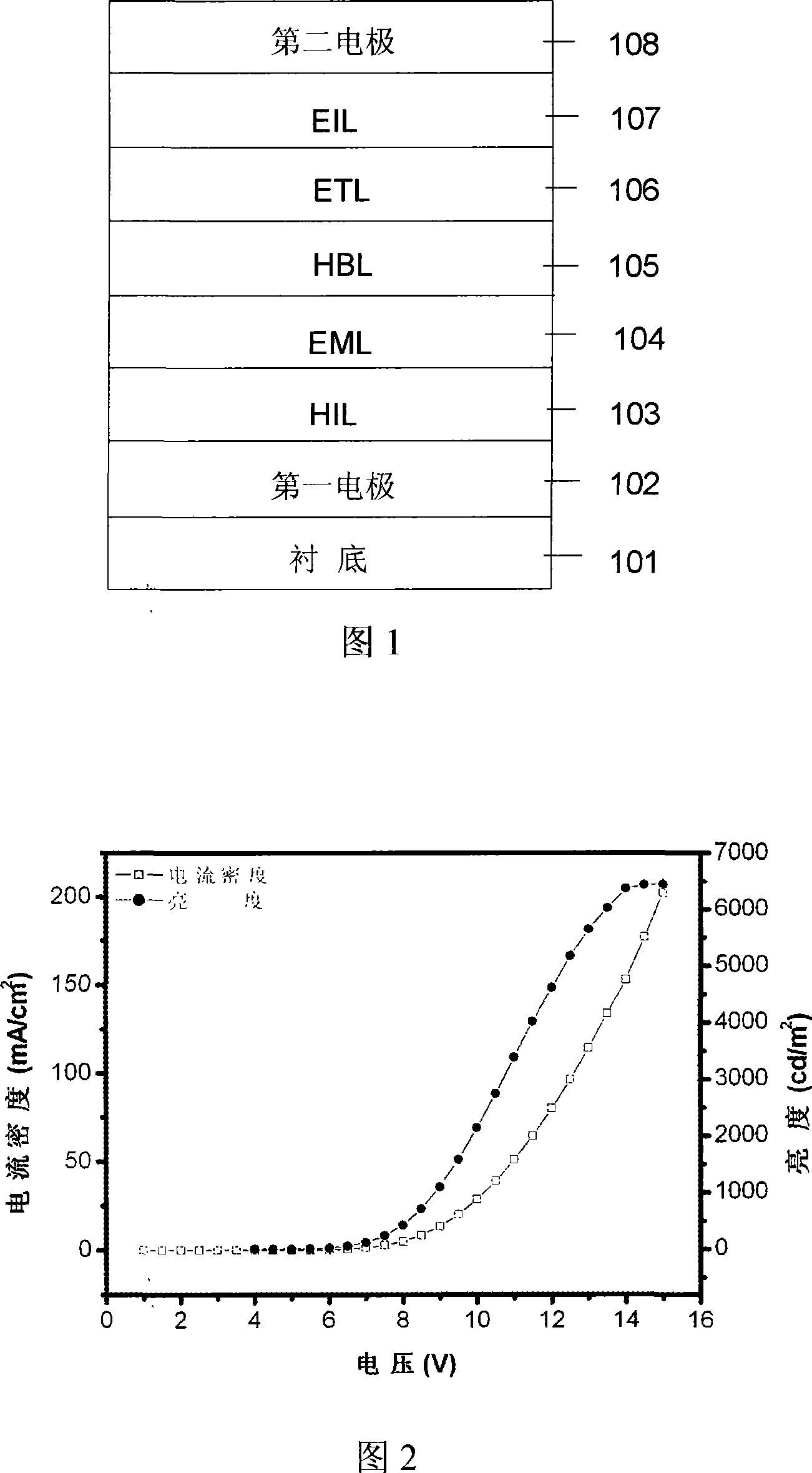
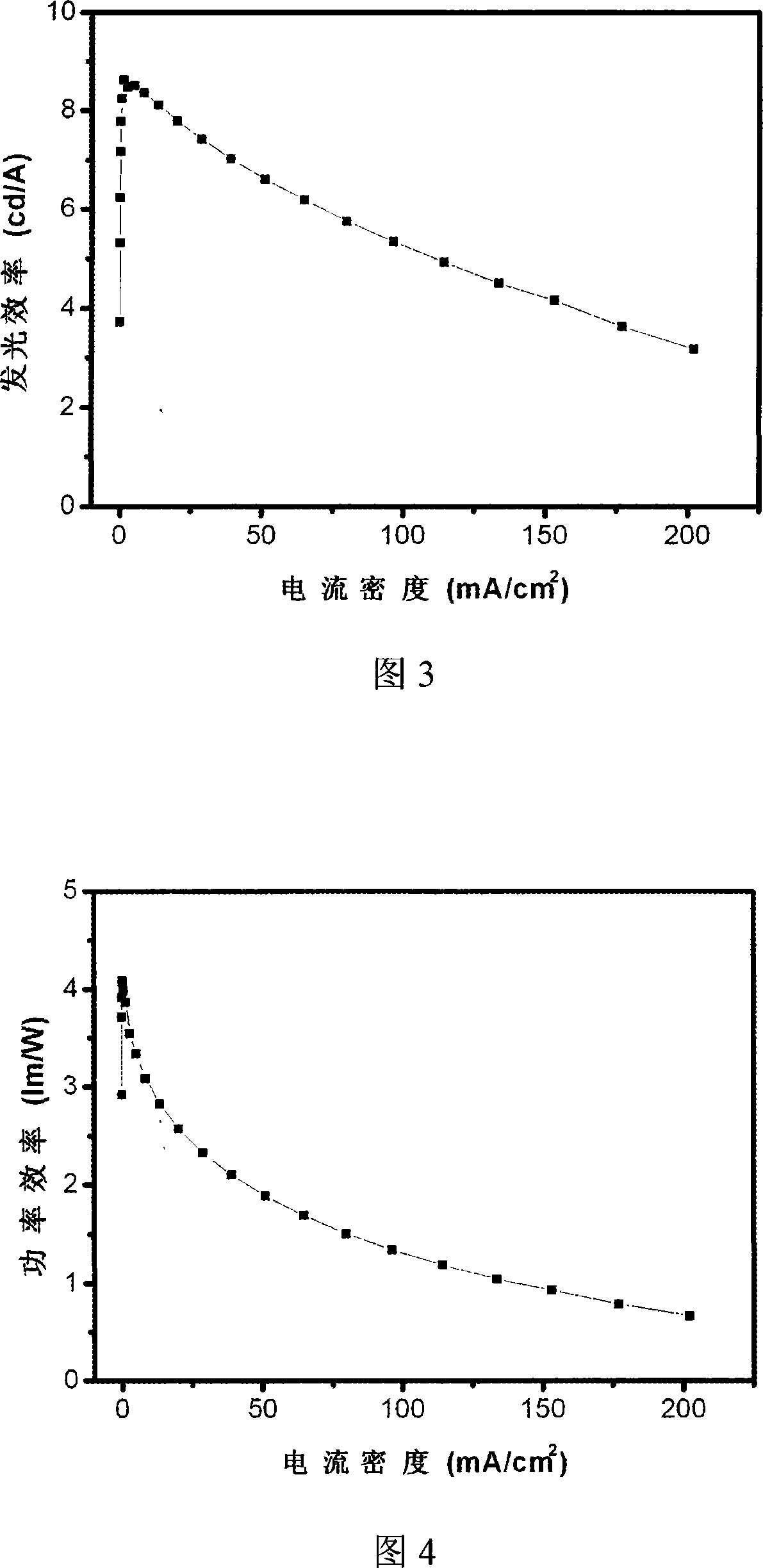
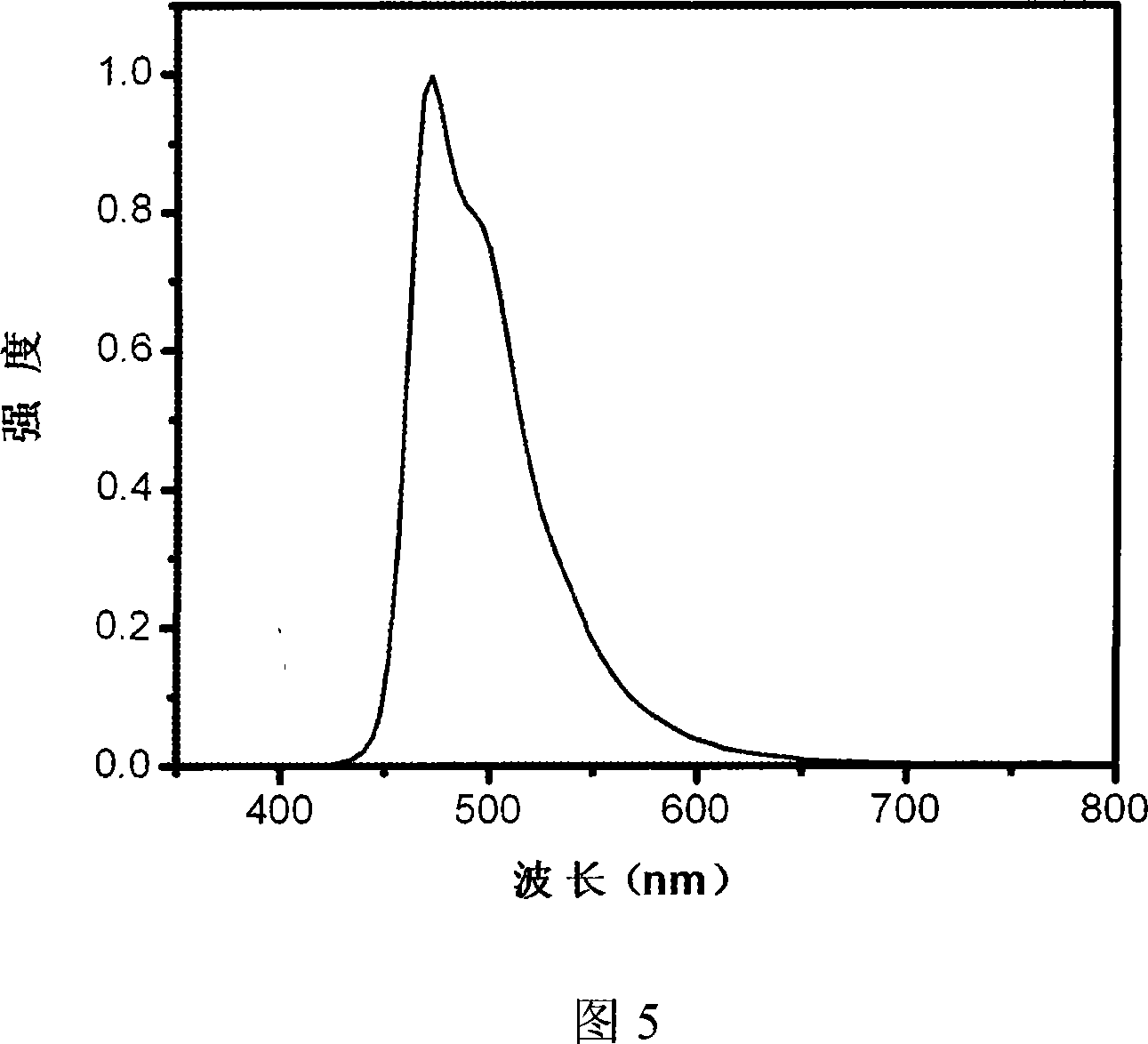
Dendritic main body material and organic electroluminescence device prepared from the same
A technology of electroluminescent devices and main materials, which is applied in the direction of electroluminescent light sources, electric light sources, electrical components, etc., and can solve problems such as device efficiency reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the synthesis of the compound of chemical formula (2)
[0058] Reaction 1:
[0059]
[0060] Under nitrogen protection, compound 3.1g (5.0mmol) of chemical formula (8), peripheral branch D 1 4.6g (16.5mmol), potassium carbonate 4.2g (30.0mmol), cuprous iodide 300mg (1.5mmol), 18-crown-6 211mg (0.8mmol) and 1,3-dimethyl-3,4,5 , a mixture of 2 mL of 6-tetrahydro-2-pyrimidinone (DMPU) was heated to 170 ° C, reacted for 24 hours, neutralized the remaining potassium carbonate with dilute hydrochloric acid, extracted with dichloromethane, washed with ammonia water to remove copper ions, and washed with saturated saline until medium properties, dried over anhydrous sodium sulfate, and the solvent was evaporated by rotary evaporation under reduced pressure, followed by column separation to obtain 4.4 g of the compound of chemical formula (2), with a yield of 82%.
Embodiment 2
[0061] Embodiment 2: the synthesis of the compound of chemical formula (3)
[0062] Reaction 2:
[0063]
[0064] Under nitrogen protection, the compound 1.6g (2.5mmol) of chemical formula (8), the peripheral branch D 2 5.9g (8.2mmol), potassium carbonate 2.1g (15.0mmol), cuprous iodide 150mg (0.75mmol), 18-crown-6 106mg (0.4mmol) and 1,3-dimethyl-3,4,5 , a mixture of 5 mL of 6-tetrahydro-2-pyrimidinone (DMPU) was heated to 190°C, reacted for 48 hours, neutralized the remaining potassium carbonate with dilute hydrochloric acid, extracted with dichloromethane, washed with ammonia water to remove copper ions, and washed with saturated saline until medium properties, dried over anhydrous sodium sulfate, and the solvent was evaporated by rotary evaporation under reduced pressure, followed by column separation to obtain 3.3 g of the compound of chemical formula (3), with a yield of 55%.
Embodiment 3
[0065] Embodiment 3: the synthesis of the compound of chemical formula (4)
[0066] Reaction 3:
[0067]
[0068] Under the protection of nitrogen, the compound 3.3g (5.0mmol) of chemical formula (9), the peripheral branch D 1 4.6g (16.5mmol), potassium carbonate 4.2g (30.0mmol), cuprous iodide 300mg (1.5mmol), 18-crown-6 211mg (0.8mmol) and 1,3-dimethyl-3,4,5 , a mixture of 2 mL of 6-tetrahydro-2-pyrimidinone (DMPU) was heated to 170 ° C, reacted for 24 hours, neutralized the remaining potassium carbonate with dilute hydrochloric acid, extracted with dichloromethane, washed with ammonia water to remove copper ions, and washed with saturated saline until medium properties, dried over anhydrous sodium sulfate, and the solvent was evaporated by rotary evaporation under reduced pressure, followed by column separation to obtain 3.4 g of the compound of chemical formula (4), with a yield of 61%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com