Method of producing ultra-fine perovskite type LaFeO*, LaMnO*, LaNiO*
A perovskite-type, ultra-fine calcium technology, applied in chemical instruments and methods, inorganic chemistry, nickel compounds, etc., can solve problems such as uneven distribution, small specific surface area, large particle size, etc., and achieve easy industrialization, specific surface area Great effect of improving physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
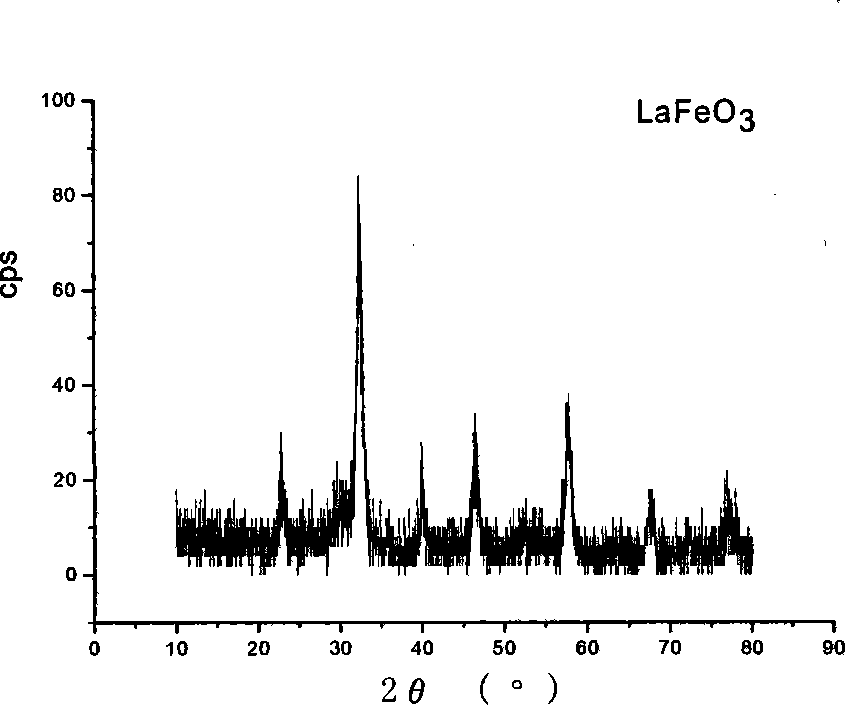
[0027] Weigh lanthanum nitrate, ferric nitrate and stearic acid at a molar ratio of 1:1:10, respectively. Under the condition of heating in an oil bath, first dissolve the stearic acid, and under constant temperature magnetic stirring, dissolve the solids of lanthanum nitrate and iron nitrate in the molten stearic acid, control the temperature at 116°C, and react for 6.0 hours to form a gel. The gel was placed in a 300°C muffle furnace and heated to burn it. Can directly obtain perovskite ultrafine powder LaFeO 3 . The XRD and morphology of the powder are shown in figure 1 with figure 2
Embodiment approach 2
[0029] Weigh lanthanum nitrate, ferric nitrate, and stearic acid at a molar ratio of 1:1:8, respectively. Under the condition of heating in an oil bath, first dissolve the stearic acid, and under constant temperature magnetic stirring, dissolve the solids of lanthanum nitrate and iron nitrate in the molten stearic acid, control the temperature at 114°C, and react for 5.0 hours to form a gel. The gel was placed in a 300°C muffle furnace and heated to burn it. Can directly obtain perovskite ultrafine powder LaFeO 3 . Particle specific surface area and morphology are shown in Table 1
[0030] 2. LaMnO 3 preparation of
[0031] Embodiment 1
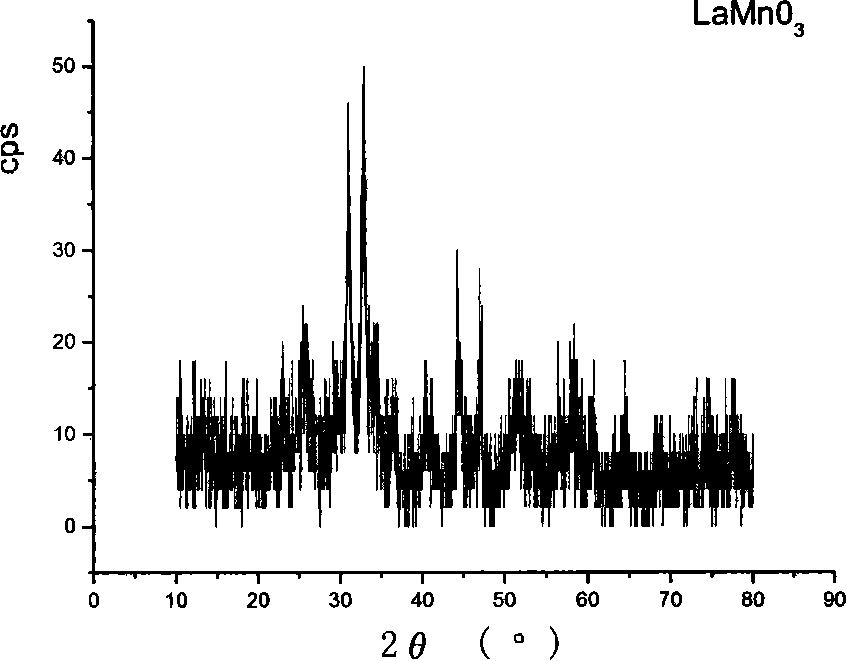
[0032] Weigh lanthanum nitrate, manganese chloride, and stearic acid at a molar ratio of 1:1:10, respectively. Under the condition of heating in an oil bath, first dissolve the stearic acid, and under constant temperature magnetic stirring, dissolve the solids of lanthanum nitrate and manganese chloride in the molten stearic acid, contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com