CDNA sequence of coding perinereis albuhitensis grube protease and amino acid sequence thereof
A technology of nereid biloba and protease is applied in the field of cDNA sequence of nereid protease and its amino acid sequence, and can solve the problems of complicated purification process, limited development and utilization, low content of protease and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Construction of a cDNA library of C. didentate and the cloning of a cDNA encoding protease:
[0046] 1. Using 2 g of Nereis cerevisiae didentate live tissue as material, total RNA was extracted using RNAiso Reagent (TaKaRa Code No.D312).
[0047] 2. Use Oligotex-dT30 mRNA Purification Kit (TaKaRa Code No.9086) to extract mRNA.
[0048] 3. Using 5 μg mRNA as a template, use the TaKaRa cDNA library construction kit to construct a cDNA library, specifically including the following steps:
[0049] (1) Use reverse transcriptase M-MLV and Oligo (dT) anchor primer to synthesize the first strand of cDNA, using 5-methyl dCTP during synthesis;
[0050] (2) Utilize RNaseH, Escherichia coli DNA polymerase and DNA ligase to synthesize the second strand of cDNA;
[0051] (3) smoothing the ends of the double-stranded cDNA using T4 DNA polymerase;
[0052] (4) Ligate with the linker containing the NotI recognition sequence, and then digest with NotI;
[0053] (5) Use DNA...
Embodiment 2
[0074] Example 2: Construction of expression vector and expression of cDNA in Escherichia coli:
[0075] 1. According to the obtained cDNA sequence, design a pair of primers
[0076] Primer 1: ACATATGCTGAATGGACCAAG, Primer 2: ACCCTCGAGGAAACCTAAAGTC
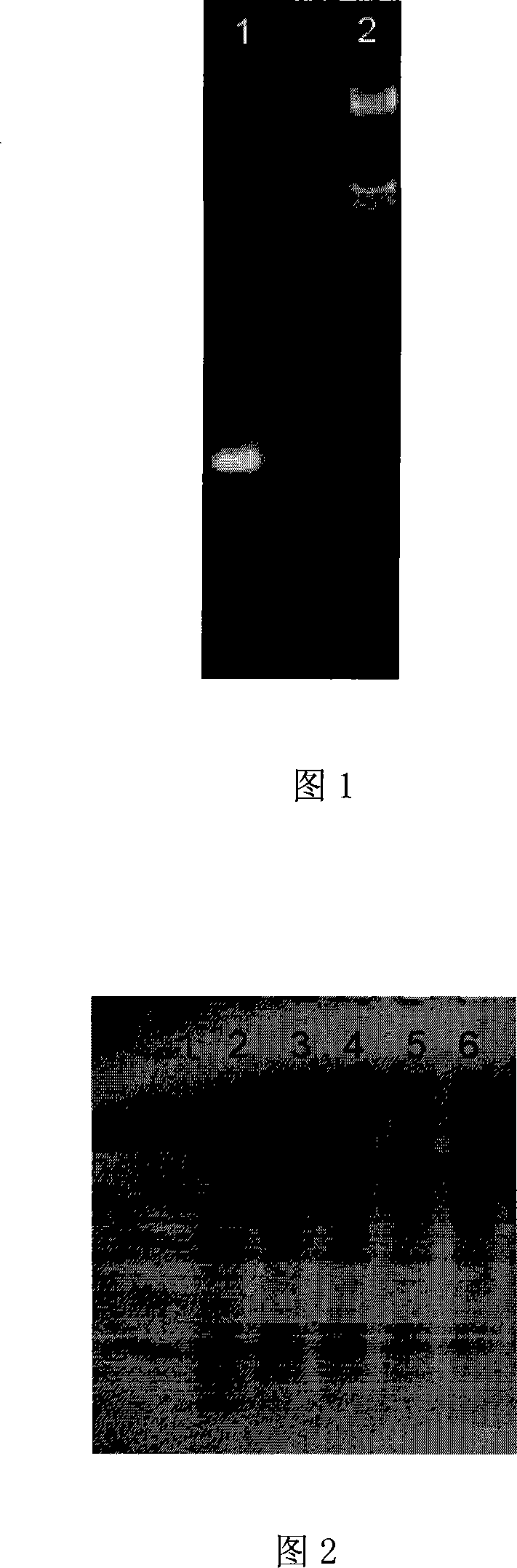
[0077] Using the cDNA of C. bidentata protease as a template, Taq DNA polymerase was used to amplify the DNA fragment without the signal peptide coding region. The amplification conditions were: denaturation at 94°C for 45 sec, annealing at 50°C for 45 sec, and extension at 72°C for 1 min. A 700bp fragment was obtained (in FIG. 1: 1. PCR product; 2. λDNA / EcoR I+HindIII).
[0078] 2. After cutting with NdeI and XhoI, clone it into the expression vector pET-15b to construct the expression vector pET-15bP, transform the expression vector into E.col iBL21(DE3), and construct the engineering bacteria.
[0079] 3. Pick a single colony of engineering bacteria, inoculate it in 20ml of LB medium containing 100μg / ml ampicillin, culture it...
Embodiment 3
[0081] Embodiment 3: Separation and purification of recombinant Bidentate nereis protease
[0082] 1. The lysate of the engineered bacteria induced to express was ultrasonically disrupted, centrifuged at 15,000×g for 30 min at 4°C, and the supernatant was taken.
[0083] 2. The supernatant flows through Ni 2+ Resin column (2.6×8cm), with 100ml washing solution (20mM, pH 8.0, 50mM imidazole, 0.5M NaCl).
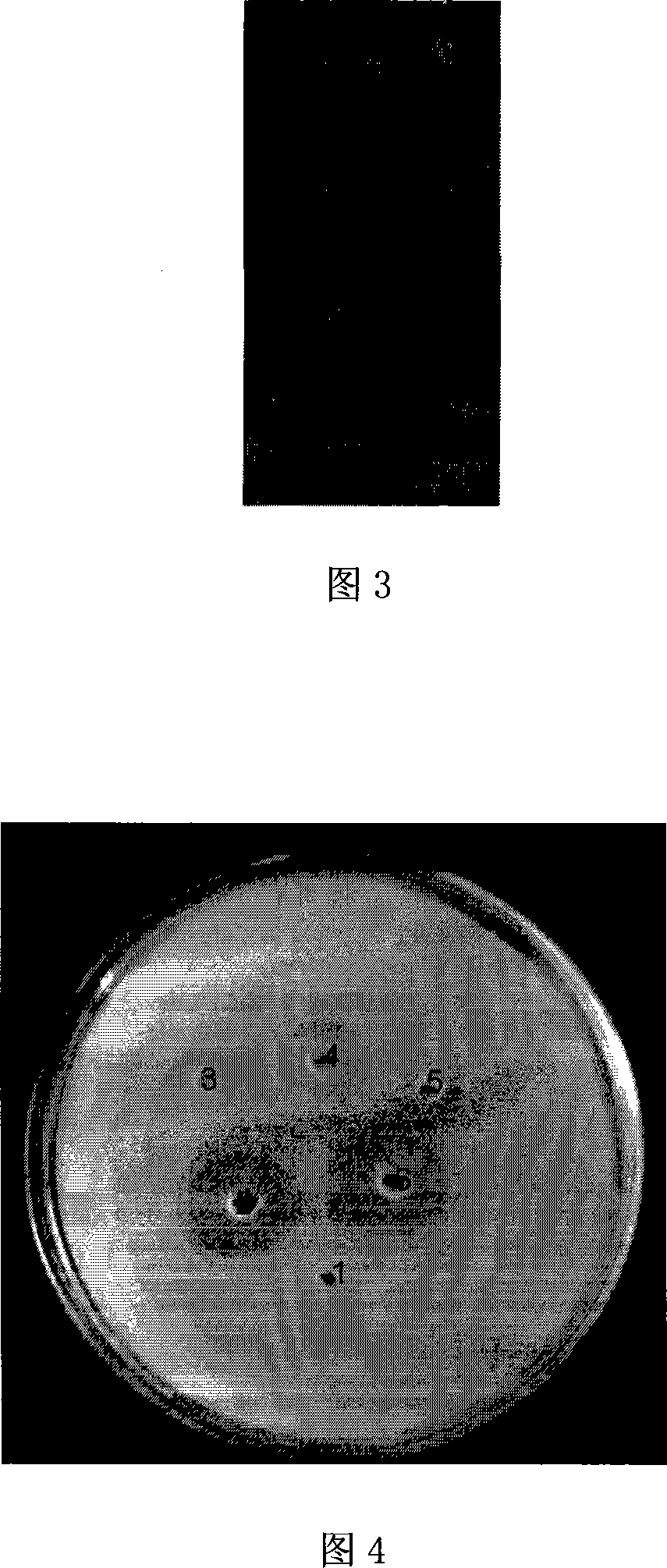
[0084] 3. Elute the binding protein with elution buffer (20mM, pH8.0, 500mM imidazole, 0.5MNaCl) to obtain the recombinant Bidentate nereis protease, the recombinant protein is 28kDa (Figure 3: 1. Medium molecular weight standard protein; 2. Unbound miscellaneous protein; 3. Recombinant Bidentate nereis protease).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com