Alpha-(aryl-or heteroaryl-methyl)-beta piperidino propanamide compounds as ORL1-receptor antagonists
A compound, heteroaryl technology, applied in the field of its medical use, can solve problems such as drug addiction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
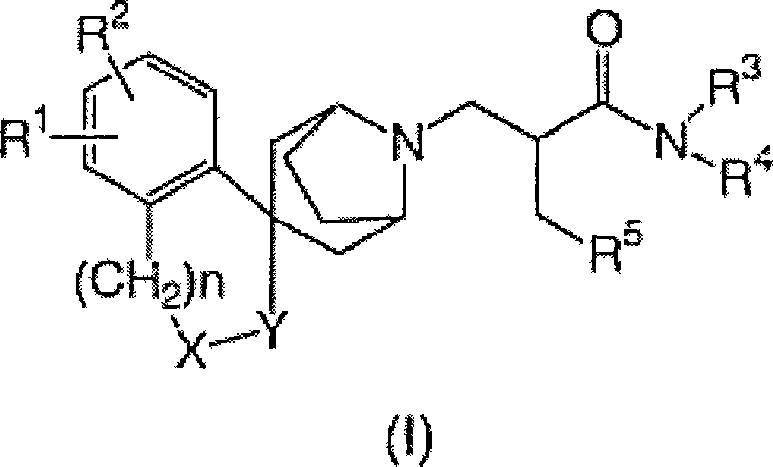
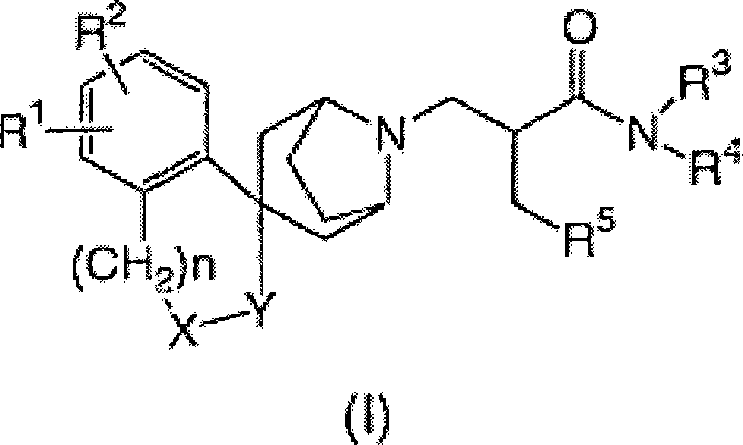
[0058] It schematically illustrates the preparation of compounds of formula (I).
[0059]
[0060] In the above formula, G represents a hydrogen atom or a hydroxyl group. R a represents an alkyl group having 1 to 4 carbon atoms. L 1 represents a leaving group. Examples of suitable leaving groups include: halogen atoms such as chlorine, bromine and iodine; sulfonates such as TfO (trifluoromethanesulfonate), MsO (methanesulfonate), TsO (toluenesulfonate) ; and similar groups.
[0061] Step 1A
[0062] In this step, where L 1 The compound of formula 1-2 representing a halogen atom can be prepared by halogenating the compound of formula 1-1 wherein G represents a hydrogen atom with a halogenating agent in a reaction-inert solvent under halogenation conditions. when R 5 When the substituent of is a hydroxyl group, the hydroxyl group is protected with a protecting group according to conventional methods.
[0063] Examples of suitable solvents include: tetrahydrofuran; ...
Embodiment 1
[0269] N,N-Dimethyl-3-(3’H,8H-spiro[8-azabicyclo[3.2.1]octane-3-1’-[2]benzofuran]-8- yl)-2-(1,3-thiazol-4-ylmethyl)propionamide citrate
[0270]
[0271] Step 1. tert-Butyl 2-(diethoxyphosphoryl)-3-(1,3-thiazol-4-yl)propanoate
[0272] 4-Methylthiazole (5.85 g, 59 mmol), N-bromosuccinimide (11 g, 62 mmol) and 2,2'-azobisisobutyronitrile (968 mg, 5.9 mmol) ) in carbon tetrachloride (200 mL) at reflux for 5 hours. After cooling, the mixture was filtered. Toluene (100 mL) was added to the filtrate, and the mixture was concentrated to obtain a toluene solution of 4-(bromomethyl)-1,3-thiazole (27 g).
[0273] To a solution of tert-butyl diethylphosphorylacetate (15.6 g, 62 mmol) in dimethylformamide (50 mL) was added sodium hydride (60% in mineral) at 0°C under nitrogen atmosphere dispersion in oil, 2.48 g, 62 mmol). After 45 minutes, a solution of 4-(bromomethyl)-1,3-thiazole in toluene (27 g) was added to the mixture. The mixture was stirred at room temperature over...
Embodiment 2
[0295] N,N-Dimethyl-3-(1H-pyrazol-1-yl)-2-(3’H,8H-spiro[8-azabicyclo[3.2.1]octane -3,1'-[2]benzofuran]-8-ylmethyl)propionamide citrate
[0296]
[0297] Step 1. Ethyl 2-(1H-pyrazol-1-ylmethyl)acrylate
[0298] A mixture of ethyl 2-(hydroxymethyl)acrylate (4.1 g, 32 mmol), pyrazole (2.6 g, 38 mmol) and potassium carbonate (11 g, 79 mmol) in acetonitrile (30 mL) Refluxed for 20 hours, quenched by addition of water (100 mL), and extracted with ethyl acetate (40 mL x 2). The combined organic layers were washed with brine, dried over magnesium sulfate, and evaporated. The residue was purified by column chromatography on silica gel, eluting with hexane / ethyl acetate (7 / 1) to give 1.0 g (18%) of the title compound as a colorless oil.
[0299] 1 H-NMR (CDCl 3 ) 7.57-7.53(1H,m), 7.48-7.45(1H,m), 6.36-6.32(1H,m), 6.28(1H,t, J=2.0Hz), 5.48-5.44(1H,m), 5.01( 2H, s), 4.24 (2H, q, J=7.1Hz), 1.30 (3H, t, J=7.1Hz).
[0300] Step 2. 3-(1H-pyrazol-1-yl)-2-(3'H,8H-spiro[8-azab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com