Preparation method of titanium barium ferrum series photocatalyst
A photocatalyst and iron-based technology, which is applied in the field of preparation of titanium-barium-iron-based photocatalysts, can solve the problem of low synthesis temperature, achieve good stability, controllable particle size, and good photocatalytic performance of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
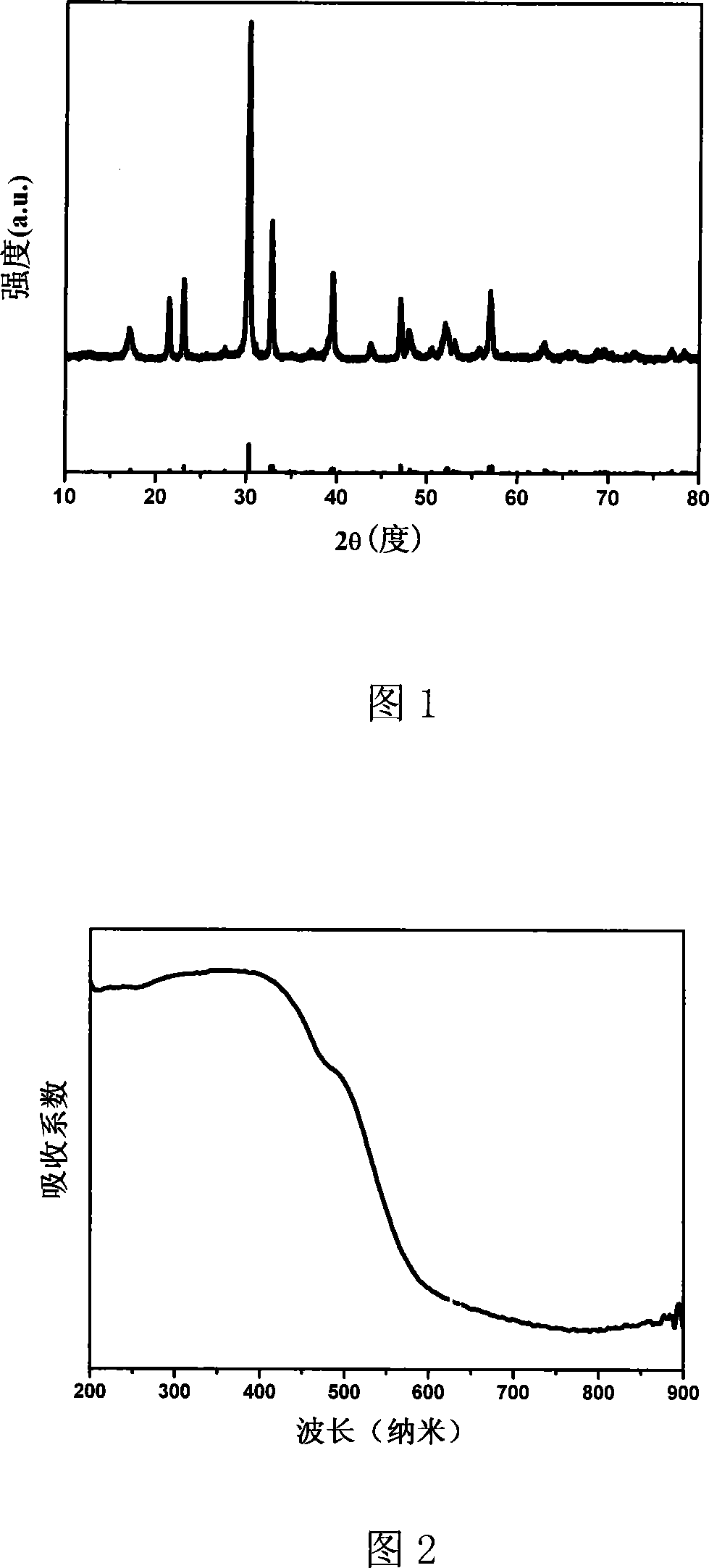
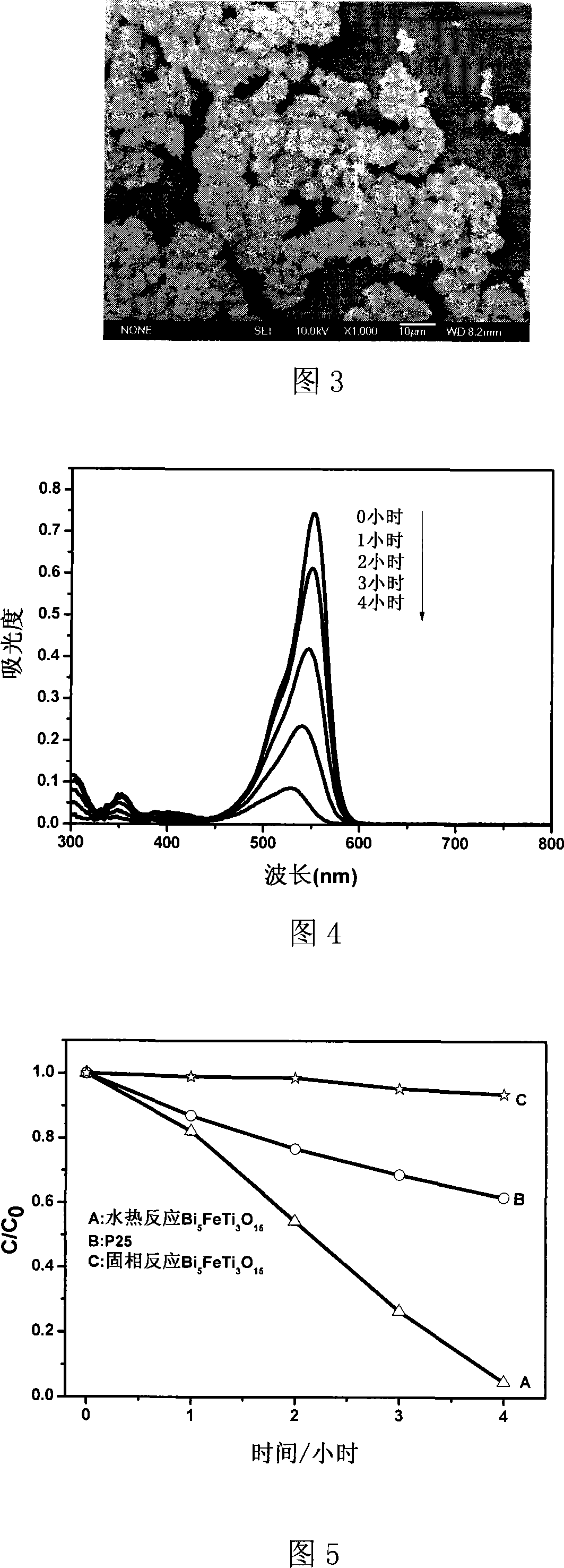
[0024] Using Bi(NO 3 ) 3 ·5H 2 O, Fe(NO 3 ) 3 9H 2 O, tetrabutyl titanate is synthesized as raw material, according to the stoichiometric ratio, weigh 2.425g Bi(NO 3 ) 3 ·5H 2 O, 0.404g Fe(NO 3 ) 3 9H 2 O, 1.02g tetrabutyl titanate was dissolved in 4mol / L nitric acid. Completely dissolve, titrate with 2mol / L NaOH until a yellow precipitate is obtained, add water to 40mL, and transfer to a 50mL hydrothermal kettle. Heating at 180°C for 60 hours. After the reaction, the obtained yellow precipitate was filtered, washed three times with deionized water and absolute ethanol, and then dried at 50°C. As shown in Figure 1, the orthogonal phase Bi of about 40nm was obtained by XRD component analysis and Debye Scherer formula calculation. 5 FeTi 3 o 15 Nanosheets. Figure 2 is the UV / Vis diffuse reflectance spectrum of the product. It can be seen from the figure that the photocatalyst has photoresponse from the ultraviolet region to the visible region, and the estimated b...
Embodiment 2
[0027] Bi 5 FeTi 3 o 15 Use BiCl 3 , FeCl 3 , TiCl 4 Synthesize as raw material, weigh 1.575g BiCl according to the stoichiometric ratio 3 , 0.162g FeCl 3 , 0.569g TiCl 4 Dissolve in 4mol / L hydrochloric acid, titrate with 2mol / L NaOH until a yellow precipitate is obtained, add water to 40mL, and transfer to a 50mL hydrothermal kettle. Heating at 180°C for 60 hours. After the reaction, the obtained yellow precipitate was filtered, washed three times with deionized water and absolute ethanol, and then dried at 50°C. The product was determined to be orthorhombic phase Bi by XRD component analysis 5 FeTi 3 o 15 , the visible light degradation RB experiment result of wavelength > 400nm is slightly lower than that of Example 1, and the degradation rate is 75%.
Embodiment 3
[0029] Bi 5 FeTi 3 o 15 Using Bi(NO 3 ) 3 ·5H 2 O, Fe(NO 3 ) 3 9H 2 O, isopropyl titanate is synthesized as a raw material, according to the stoichiometric ratio, weigh 2.425g Bi(NO 3 ) 3 ·5H 2 O, 0.404g Fe(NO 3 ) 3 9H 2 O, 0.852g of isopropyl titanate was dissolved in 5mL of glacial acetic acid. Titrate with 1mol / L KOH until a yellow precipitate is obtained, add water to 40mL, and transfer to a 50mL hydrothermal kettle. Heating at 180°C for 60 hours. After the reaction, the obtained yellow precipitate was filtered, washed three times with deionized water and absolute ethanol, and then dried at 50°C. The product was determined to be orthorhombic phase Bi by XRD component analysis 5 FeTi 3 o 15 , the visible light degradation RB experiment result of wavelength>400nm is lower than embodiment 1, and the degradation rate is 67%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com