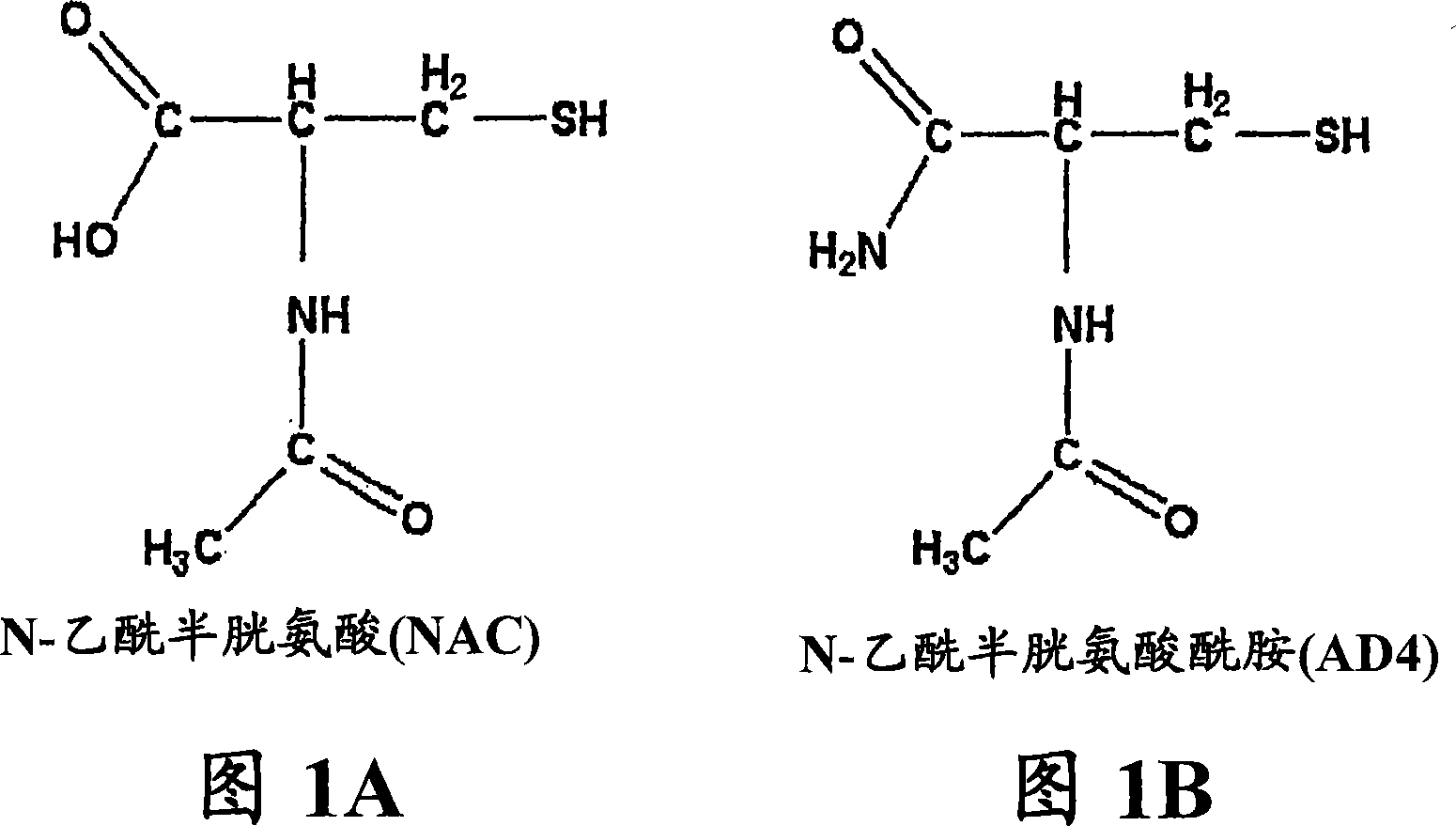
N-acetylcysteine amide (NAC amide) for the treatment of diseases and conditions associated with oxidative stress
A amide and disease technology, applied in the field of antioxidant treatment of mammals including human diseases, can solve toxic and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
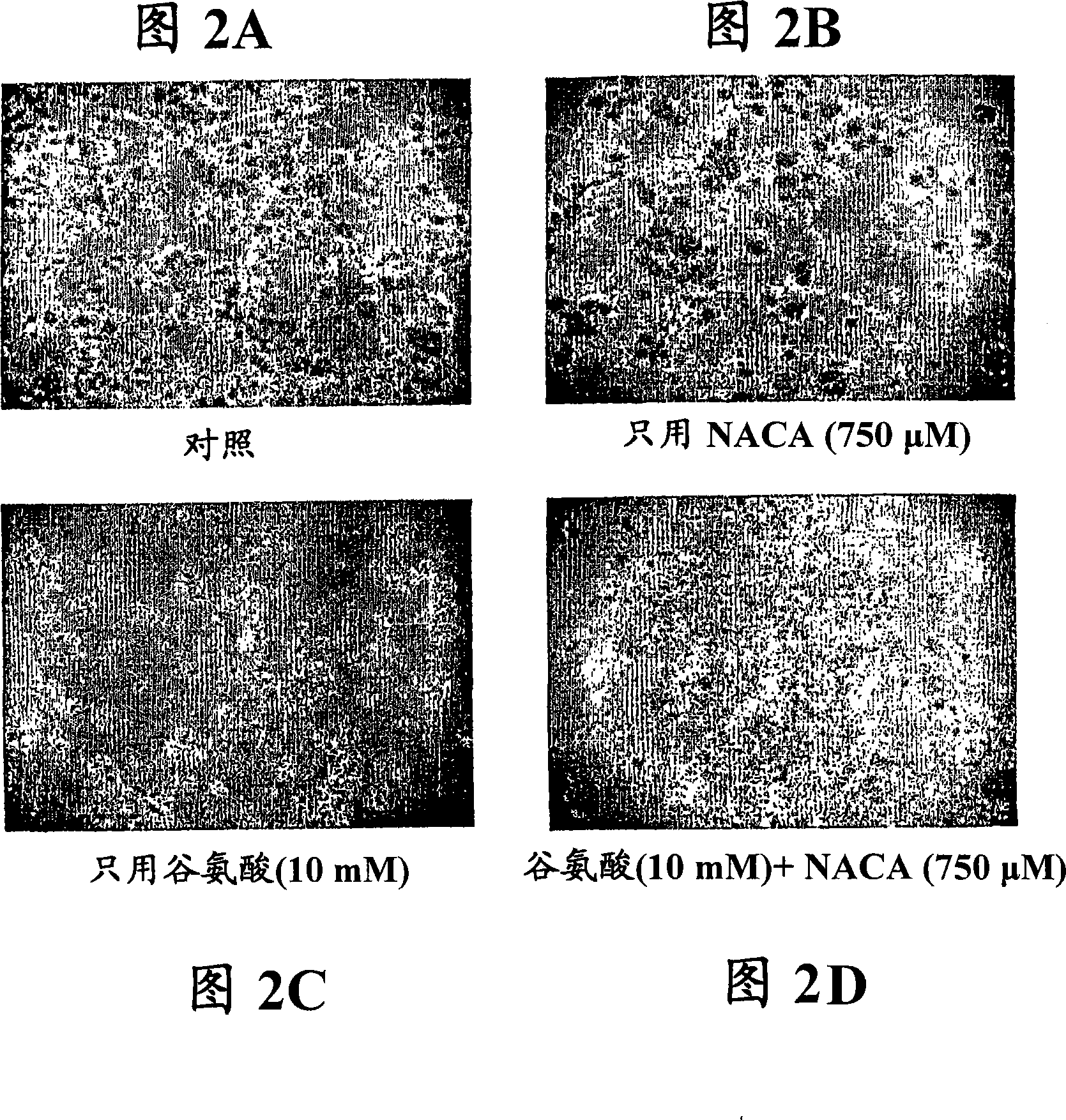
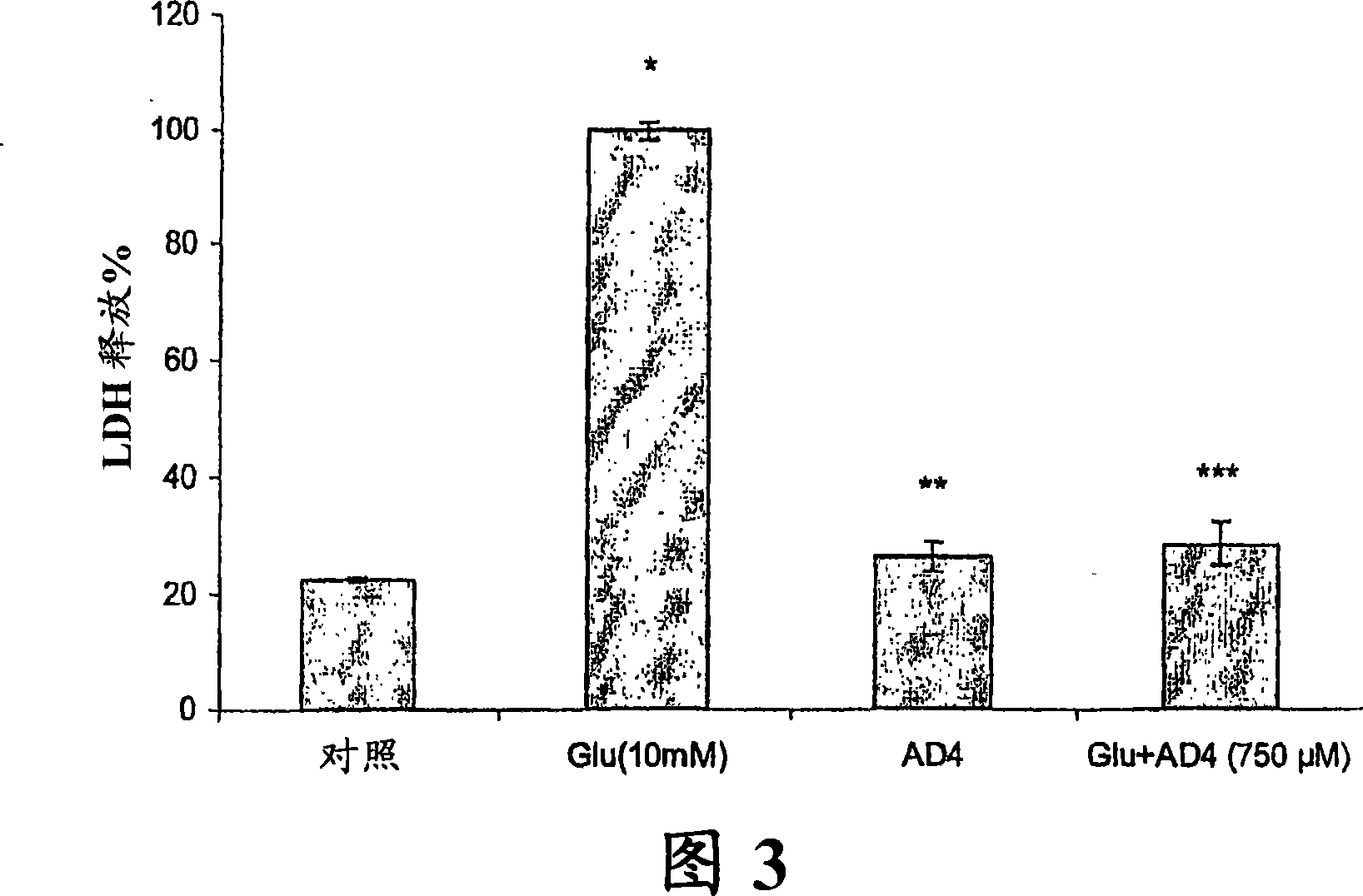
[0218] In this example, the protective effect of NAC amide against glutamate-induced oxidative toxicity in PC12 cells was assessed.
[0219] Materials and methods: N-(1-pyrenyl)-maleimide (NPM) was purchased from Aldrich (Milwaukee, WI, USA). N-acetyl cysteine amide was purchased from Novia Pharmaceuticals, (Israel). High performance liquid chromatography (HPLC) grade solvents were purchased from Fisher Scientific (Fair Lawn, NJ). All other chemicals were purchased from Sigma (St. Louis, MO, USA).
[0220] Cell culture and toxicity studies: stock cultures of PC12 cells purchased from ATCC were grown in 75 cm 2 Grow in RPMI 1640 supplemented with 10% (v / v) heat-inactivated horse serum and 5% (v / v) fetal bovine serum in tissue culture flasks, add 1% (v / v) penicillin and strepto Mycin. Maintain the medium at 37 °C with 5% CO 2 in a humid atmosphere. Cells were passaged twice a week. Unless otherwise stated, all experiments used Dulbecco's modified Eagle's medium (DMEM) a...
Embodiment 2
[0241] This example detects the radioprotective effect of NAC amide. To assess the protective effect of NAC amide against radiation exposure, the radioprotective effect of NAC amide was compared with that of NAC in terms of increasing GSH levels and restoring oxidative stress parameters to their normal control values.
[0242] Animal studies: Rats were irradiated at the Radiation Oncology Department of the Phelps County Regional Medical Center, Rolla, MO, USA, using a 16 MeV beam generated by a Varian linear accelerator, model Clinac 1800, according to standards of humane laboratory animal practice. Use 20×20 or 25×25cm to irradiate the field, and check the output coefficient once a week. 12 animals were divided into 4 groups, each group consisted of 3 animals (control, XRT, NACamide+XRT and NAC+XRT groups). The radiation (XRT) control group received whole-body irradiation of 6Gy 16MeV electrons. The NACamide+XRT group received 500 mg / kg / day of NACamide shortly before irradi...
Embodiment 3
[0320] This example describes a treatment regimen suitable for humans. NACamide was administered between meals (on an empty stomach), 1-3 grams per day, in 2 divided doses. Encapsulated NAC amide is suitable for administration (NAC amide formulation containing 500 mg NAC amide and optionally 250 mg USP grade crystalline ascorbic acid and not more than 0.9 mg magnesium stearate (NF grade) in an OO-type gelatin capsule). Although the human body produces large amounts of glutathione, administration of exogenous NAC amide is also expected to produce a dose-response effect in patients.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com