Method for synthesizing spirodiclofen
A synthesis method and a technology for spirodiclofen, applied in the field of spirodiclofen, can solve problems such as being unsuitable for industrialized production, and achieve the effects of increasing the reaction yield and simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、2
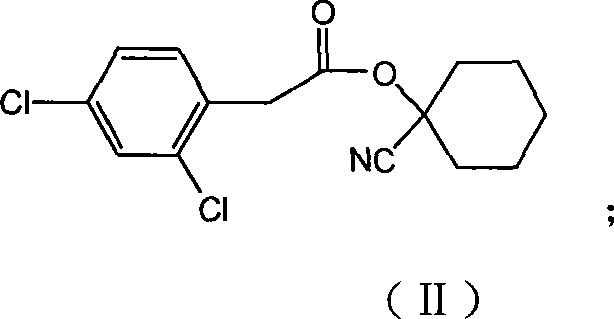
[0043] Embodiment 1,2, the preparation of 4-dichlorophenylacetic acid-1-cyanocyclohexyl ester (compound II):
[0044] Add 7.9g (63.4mmol) of the above-mentioned 1-cyanocyclohexanol, 13.9g (175mmol) of pyridine, and 60mL of dichloromethane into the reaction flask, and add 20.0 The dosing of g (89.6mmol) 2,4-dichlorophenylacetyl chloride and 40mL dichloromethane. After the dropwise addition was completed for about 1h, the stirring reaction was continued for 12h.
[0045] After the reaction was finished, concentrate, add 100mL 3% (V / V) HCI, extract three times with ethyl acetate, the ethyl acetate layer was washed with 5% (W / W) Na 2 CO 3 The solution was washed twice and finally with water. Anhydrous Na 2 SO 4 Drying and concentration gave 28.9 g of a brown paste, which was washed with tert-butanol to give 13.4 g of the product, yield Y=77.2%.
Embodiment 2、1
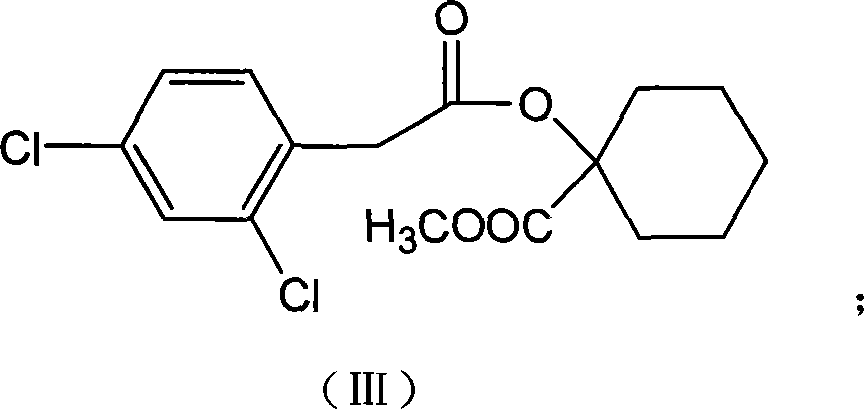
[0046] Example 2, the preparation of 1-[2-(2,4-dichloro-phenyl)-acetoxy]-cyclohexanemethylcarboxylate (compound III):
[0047] Add 10.0g (32.2mmol) 2,4-dichlorophenylacetic acid-1-cyanocyclohexyl and 80ml concentrated sulfuric acid methanol solution after washing with tert-butanol obtained in the above-mentioned embodiment 1 and heat and stir to reflux Reaction 20h. This 80ml concentrated sulfuric acid methanol solution is made by distilling 8mL of concentrated sulfuric acid with methanol.
[0048] After the reaction was completed, most of the methanol was evaporated, and 50 mL of water was added after cooling, and the product was extracted 3 times with ethyl acetate, and the combined ethyl acetate layers were washed with 5% (W / W) NaHCO 3 solution was washed 3 times, and then washed with water, anhydrous Na 2 SO 4 Drying and concentration gave 8.6 g of product, Y=77.8%.
Embodiment 3
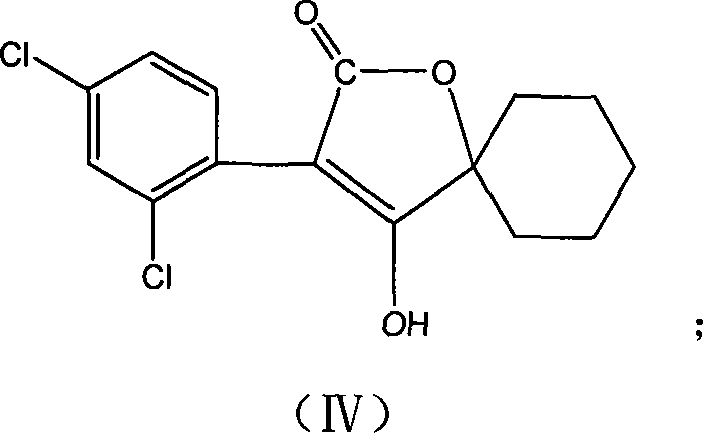
[0049]Example 3, Preparation of 3-(2,4-dichlorophenyl)-2-oxo-1-oxaspiro[4.5]-dec-3-ene 4-ol (compound IV):
[0050] Add 8.0g (23.1mmol) 1-[2-(2,4-dichloro-phenyl)-acetoxyl group]-cyclohexanemethyl carboxylate, 1.8g ( 16mmol) magnesium ethylate and 200mL of ethanol, heated to reflux for 12h.
[0051] After the reaction, evaporate most of the solvent; then add 5% hydrochloric acid to adjust the pH to acid; then extract with ethyl acetate, then wash with water, anhydrous Na 2 SO 4 Drying and concentration yielded 5.6 g of solid product, Y=77.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com