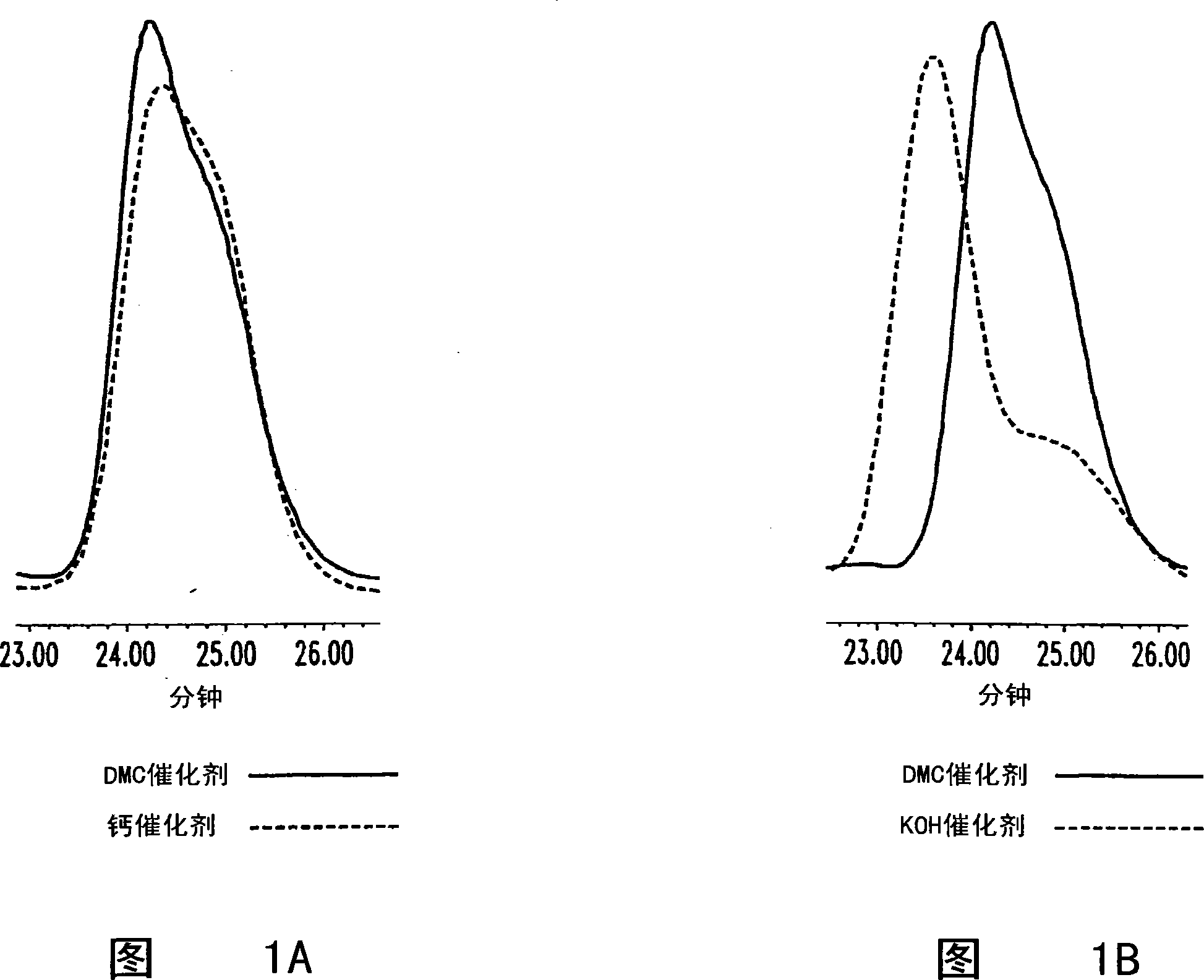
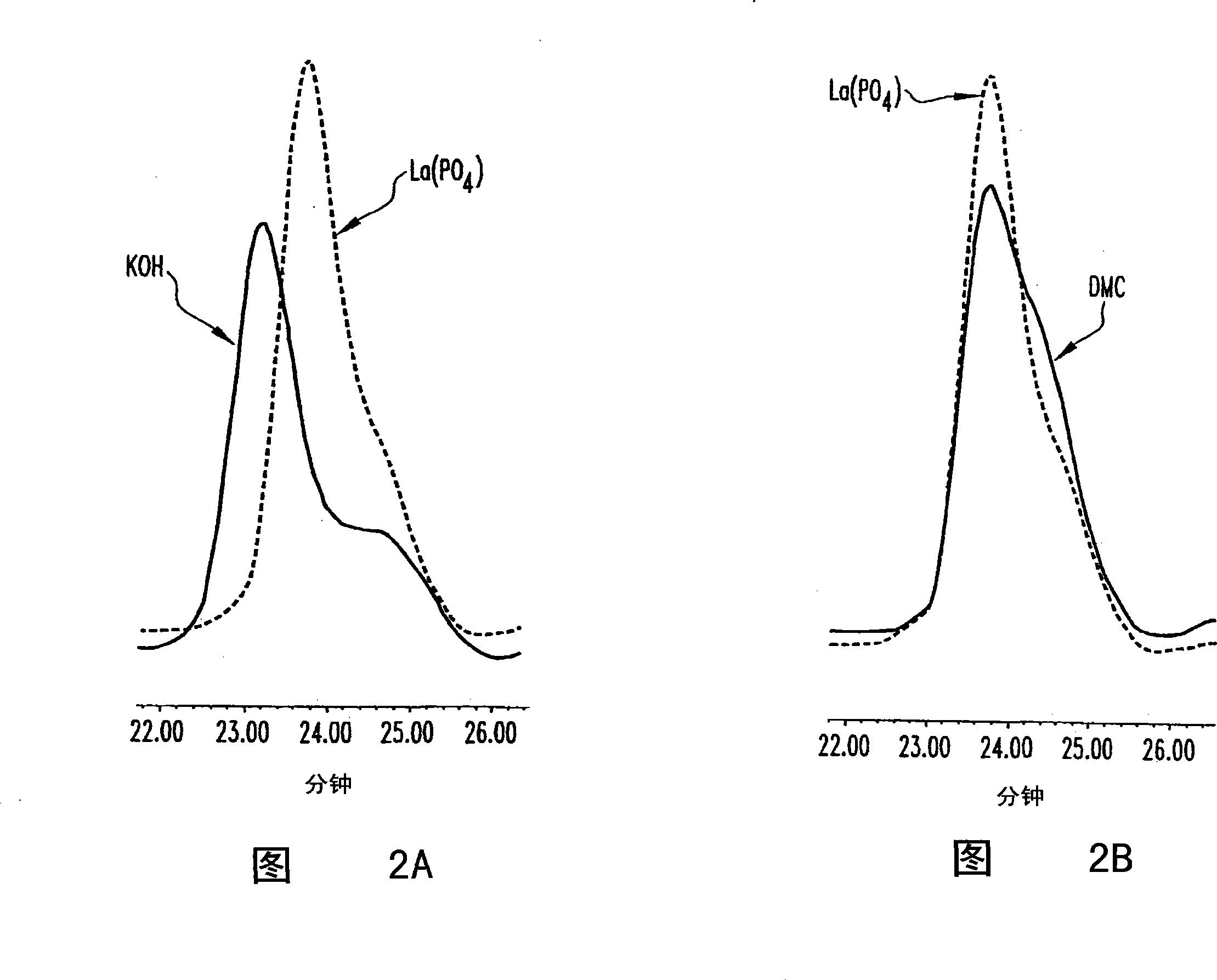
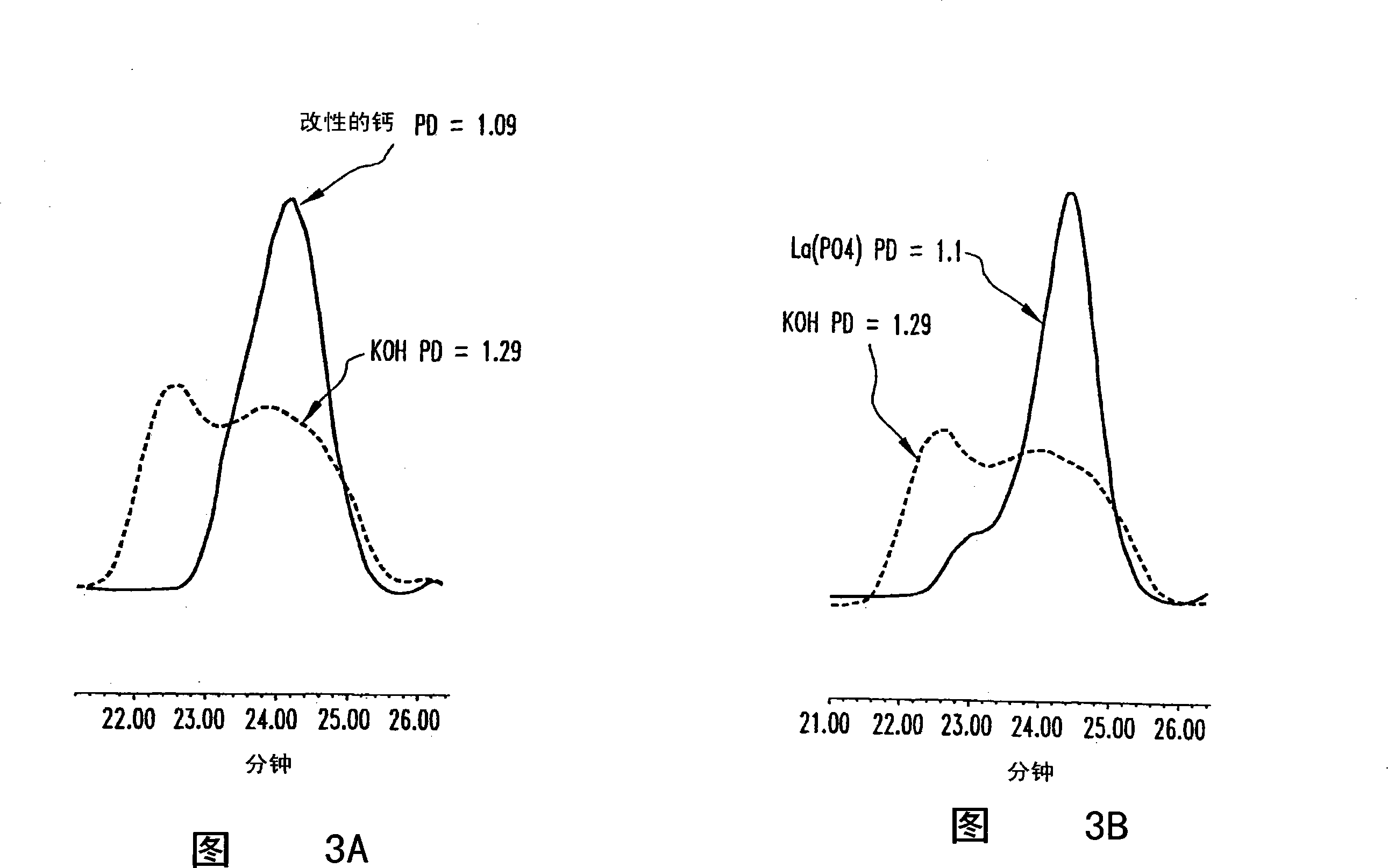
High productivity alkoxylation processes
A technology of polyalkoxylation and polyoxyalkylene, applied in the field of polymerization, can solve the problems of increasing complexity and slow kinetic rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In this example, calcium-based catalysts were prepared without aluminum alkoxides used in the preparation. A 250 ml three-necked flask reaction vessel was purged with nitrogen, and calcium hydroxide (9.75 g) was added together with TOMADOL 23-3 (106.2 g). The mixture was stirred at room temperature and 2-ethylhexanoic acid (2.23 g) was added by rapid dropwise addition over about 3 minutes. The reactants in the flask were heated to 30° C. in about 1 hour under stirring, and a vent was connected to the atmosphere through a reflux condenser. The contents of the flask were maintained at 30°C for an additional hour. Concentrated sulfuric acid (2.46 grams) was added to the flask at a rate of about 1 drop every 3 seconds. The contents of the flask were heated at 30°C for 15 minutes and an additional 2.46 grams of sulfuric acid was added at a rate of about 1 drop every 3 seconds. A reflux condenser was added to the three-necked flask and the contents of the flask were heated...
Embodiment 2
[0056] In this example, as described on page 31 of WO 04 / 18096 (Example B Reverse Phase Addition (LAPO)), in a 500 ml Lanthanum phosphate catalyst was prepared in a glass reactor. Lanthanum carbonate (15.6 g, 0.032 mol) was added to distilled water degassed with 100 mL of nitrogen (the solution was a slurry). A phosphoric acid solution was prepared by mixing 8.25 grams of 85% phosphoric acid (0.071 moles) into 100 milliliters of nitrogen degassed distilled water. Phosphoric acid was added to the carbonate solution at 25°C within 30 minutes. The solution was heated to 100°C for 2.5 hours. The product was cooled to room temperature and the solid was filtered.
[0057] The solid was transferred to a glass container and 250 mL of nitrogen-degassed distilled water was added at 50 °C for 30 min under rapid stirring. The product was cooled to about 25°C and filtered. The solid was transferred to a reactor and treated with ammonium hydroxide (5 mL, 10N) in water (250 mL). The co...
Embodiment 3
[0059] This example evaluates the modified calcium catalyst using a catch-up kinetic test. To the reactor was added nonylphenol 9.5 EO half batch (200 g), NEODOL 25 (200 g) and the catalyst mixture prepared in Example 1 DMC catalyzed C 12 3EO (14 g), heated to 100°C. The mixture was stripped at 100°C for 30 minutes, heated to 150-160°C and nitrogen blanketed to 30 psia. Ethylene oxide (286 g, 6.5 moles) was added over 2-4 hours. The total pressure (ethylene oxide and nitrogen) was maintained at less than about 60 psia. The mixture was digested at 150-160 °C until the baseline remained stable and the digestion process was continued for an additional 30 min. The mixture was cooled to 130°C, stripped for 20 minutes, and cooled to discharge temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com