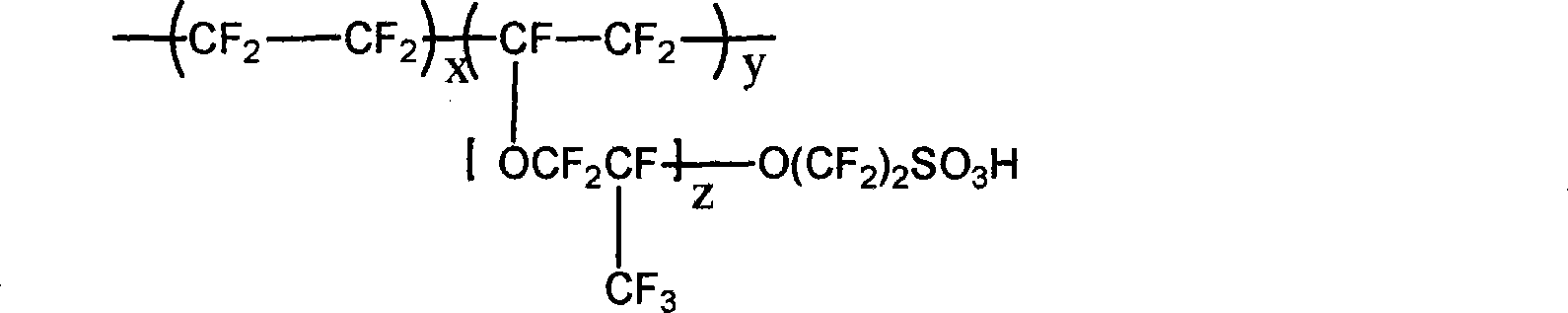Sulfonated polyarylether with sulfonic group at side chain naphthalene ring and preparation method thereof
A technology of sulfonated polyarylether and sulfonic acid group, which is applied in the field of sulfonated polyarylether and its preparation, can solve the problems of difficult control of sulfonation position and degree of sulfonation, easy occurrence of various side reactions, and difficulty in industrialization, etc. , to achieve excellent chemical physical properties and mechanical properties, ensure chemical stability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
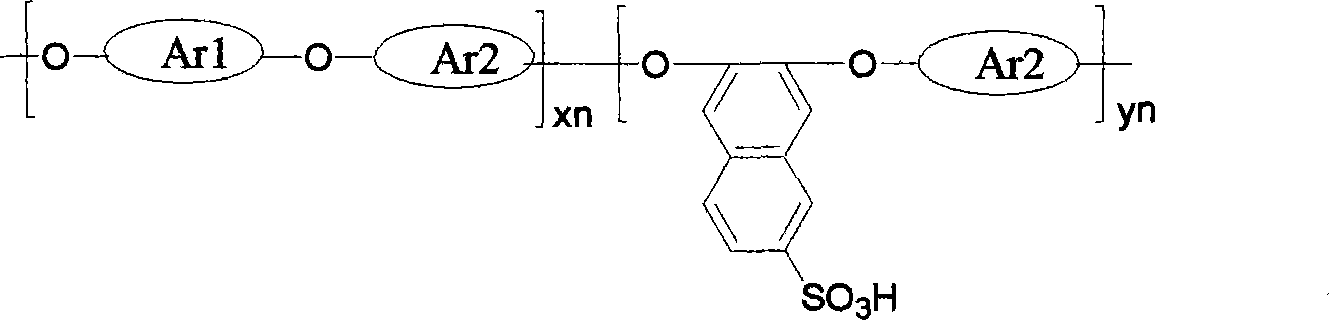
[0027] A sulfonated polyaryl ether with sulfonic acid group on the side chain naphthalene ring, its structural formula is:
[0028]
[0029] among them for for
[0030] The preparation method of the sulfonated polyaryl ether is:
[0031] a. The amount of the substance is 20mol and the structural formula is The sulfonated bisphenol monomer, the amount of substance is 20mol, the structural formula is The bisphenol monomer, the amount of the substance is 40mol, the structural formula is The difluoromonomer and 44mol of anhydrous potassium carbonate are mixed in 80L of polar aprotic solvent N,N-dimethylacetamide (DMAC);
[0032] b. Add 160L of toluene, heat up to 135°C for 3 hours under the protection of nitrogen, and distill the toluene out;
[0033] c. The temperature is increased to 160°C and reacted for 12 hours to obtain the sulfonated polyaryl ether with sulfonic acid group on the side chain naphthalene ring.
Embodiment 2
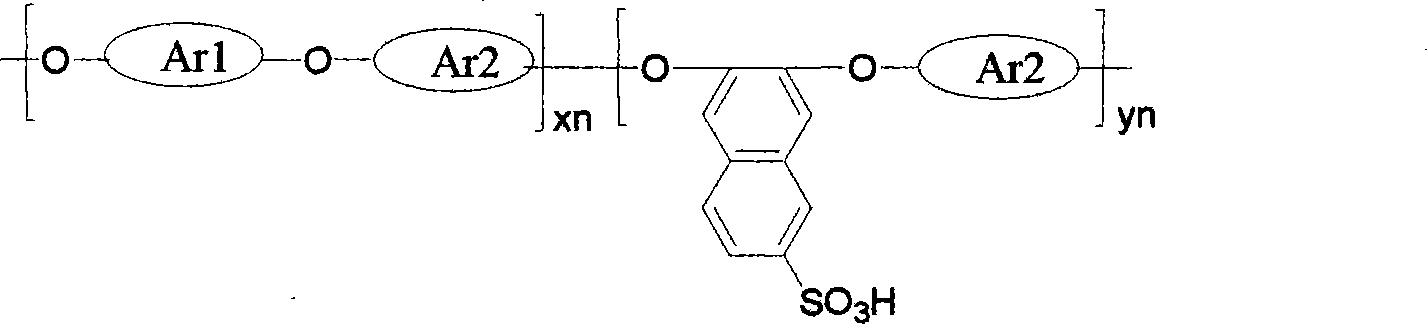
[0035] A sulfonated polyaryl ether with sulfonic acid group on the side chain naphthalene ring, its structural formula is:
[0036]
[0037] The preparation method of the above-mentioned sulfonated polyarylether is:
[0038] a. The amount of the substance is 145 mol and the structural formula is The sulfonated bisphenol monomer, the amount of substance is 145mol The structural formula is The bisphenol monomer, the amount of substance is 290mol, the structural formula is Difluoromonomer and 870mol of anhydrous potassium carbonate are mixed in 2030L of polar aprotic solvent N,N-dimethylformamide (DMF);
[0039] b. Add 2900L of toluene, heat up to 135°C for 4 hours under the protection of nitrogen, and distill the toluene out;
[0040] c. The temperature is increased to 160° C. and reacted for 24 hours to obtain a sulfonated polyaryl ether with a sulfonic acid group on the side chain naphthalene ring.
Embodiment 3
[0042] A sulfonated polyaryl ether with sulfonic acid group on the side chain naphthalene ring, its structural formula is:
[0043]
[0044] The preparation method of the above-mentioned sulfonated polyarylether is:
[0045] a. The amount of the substance is 60 mol and the structural formula is The sulfonated bisphenol monomer, the amount of substance is 40mol, the structural formula is The bisphenol monomer, the amount of substance is 100mol, the structural formula is The difluoromonomer and 200mol of anhydrous potassium carbonate are mixed in 500L of polar aprotic solvent 1-methyl-2-pyrrolidone (NMP);
[0046] b. Add 700L of toluene, heat up to 135°C for 4 hours under the protection of nitrogen, and distill the toluene out;
[0047] c. Raise the temperature to 160°C and react for 18 hours to obtain the sulfonated polyaryl ether with the sulfonic acid group on the side chain naphthalene ring.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com