Preparation method of high-purity diiodosilane
A technology of diiodosilane and iodosilane, which is applied in the field of preparation of high-purity diiodosilane, can solve the problems of expensive phenylsilane, difficult operation, slow reaction kinetics, etc., and achieve easy scale-up production and strong operation controllability , The effect of high product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The embodiment of the present invention proposes a preparation method of high-purity diiodosilane, comprising the following steps:
[0032] Step S1: wash and dry the containers and pipelines involved in the process, and purge them with inert gas; under the protection of inert gas, use solvent ethers as solvents, control the reaction temperature at 0~25°C under stirring conditions, and Add lithium aluminum tetrahydrogen to solvent ethers, then add phenyldichlorosilane dropwise, and stir for 2~4 h after the phenyldichlorosilane is added dropwise to obtain a mixture of phenylsilanes; in the reaction of preparing diiodosilane In the process, the reactants and products are very easy to react with moist air and water. The present invention ensures the chemical stability of phenylsilane and diiodosilane during the whole reaction process through the protection of inert gas.
[0033] Further, in a preferred embodiment of the present invention, the dosage ratio of solvent ethers,...
Embodiment 1
[0047] The present embodiment proposes a kind of preparation method of high-purity diiodosilane, and it comprises the following steps:
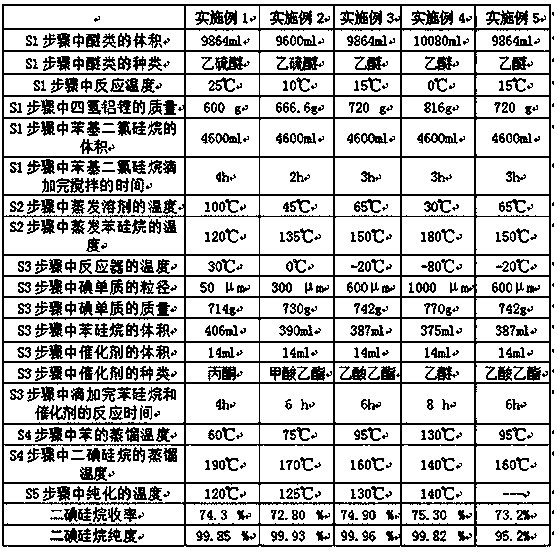
[0048] Step S1: Wash and dry the first reactor, collector, second reactor, purifier and corresponding connecting pipelines, etc., and purging with nitrogen. Under the protection of nitrogen atmosphere, add 9864ml of ethyl alcohol to the first reactor Thioether is used as a solvent, and the reaction temperature is controlled to be 25°C under stirring conditions. 600g of lithium tetrahydrogen aluminum is added to the reactor, and then 4600ml of phenyldichlorosilane is added dropwise. After the dropwise addition of phenyldichlorosilane is completed, After stirring for 4 h, a mixture of phenylsilanes was obtained.
[0049] Step S2: raise the temperature of the reactor to heat the phenylsilane mixture, distill diethyl sulfide to 100° C., continue to raise the temperature of the reactor to 120° C., distill the phenylsilane, and collect the phenylsi...
Embodiment 2
[0056] The present embodiment proposes a kind of preparation method of high-purity diiodosilane, and it comprises the following steps:
[0057] Step S1: Wash and dry the first reactor, collector, second reactor, purifier and corresponding connecting pipelines, etc., and purging with nitrogen. Under the protection of nitrogen atmosphere, add 9600ml of ethyl alcohol to the first reactor. Thioether is used as a solvent, and the reaction temperature is controlled at 10°C under stirring conditions. Add 666.6g of lithium aluminum tetrahydride to the reactor, and then add 4600ml of phenyldichlorosilane dropwise until the phenyldichlorosilane is added dropwise. , and stirred for 2 h to obtain a mixture of phenylsilanes.
[0058] Step S2: raise the temperature of the reactor to heat the phenylsilane mixture, distill diethyl sulfide to 45° C., continue to raise the temperature of the reactor to 135° C., distill the phenylsilane, and collect the phenylsilane in the collector.
[0059] S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com