High temperature proton exchange film for fuel cell and method for making same
A proton exchange membrane, fuel cell technology, applied in fuel cells, fuel cell parts, solid electrolyte fuel cells, etc., can solve the problems of decreased proton concentration, no alcohol barrier properties, and decreased conductivity of the diaphragm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
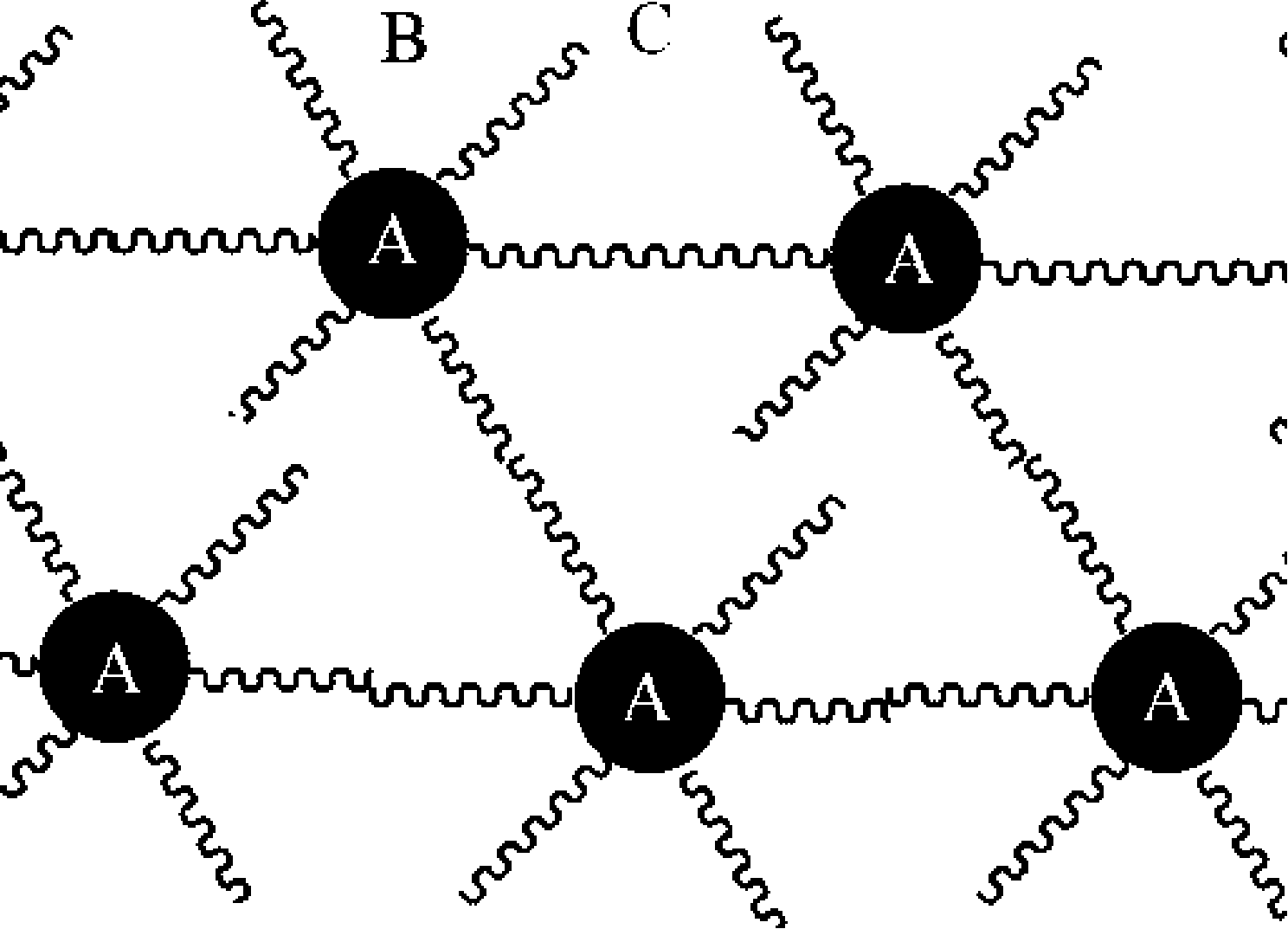
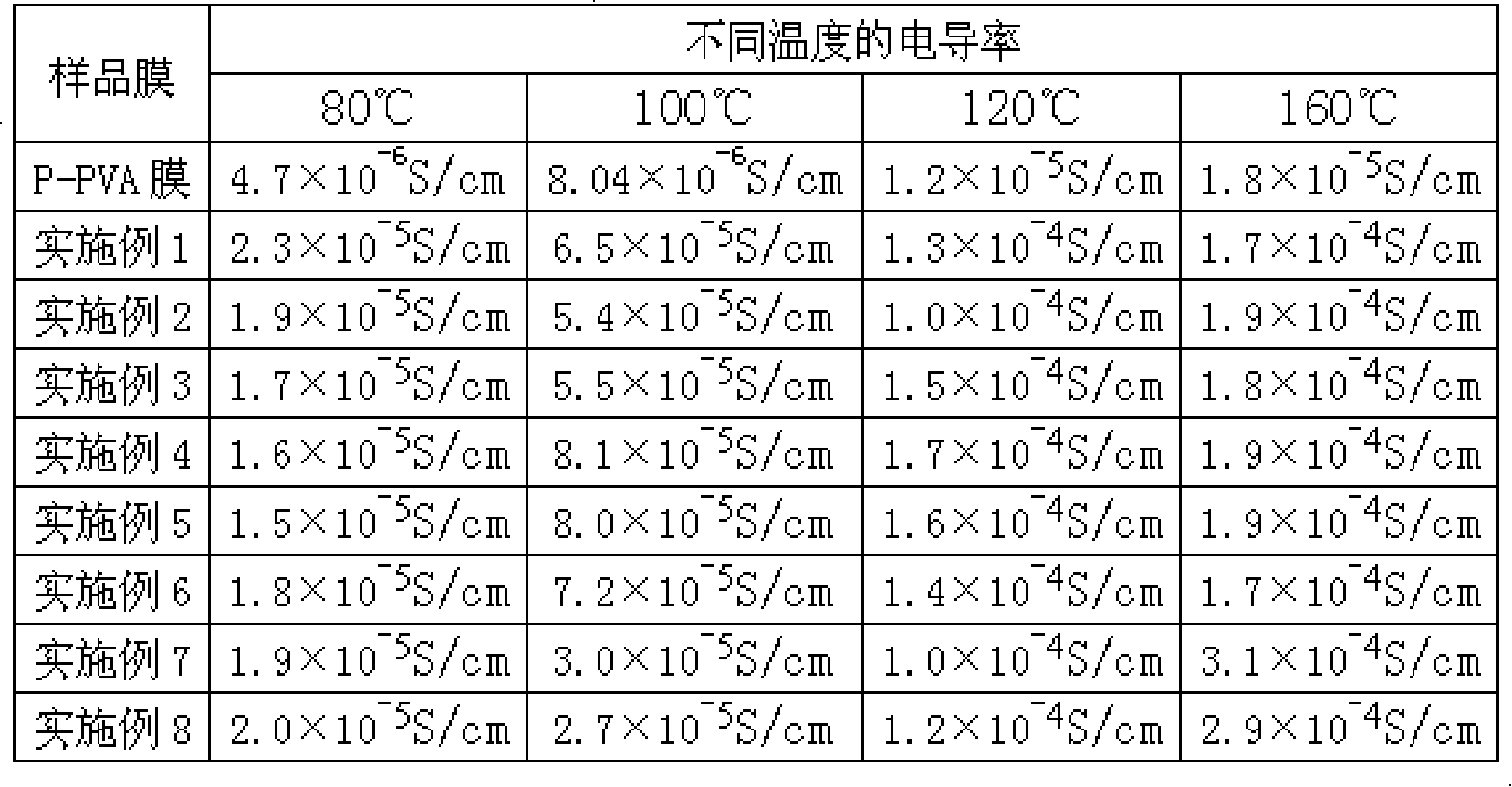
Image
Examples
Embodiment 1
[0046] The preparation (SFS) of embodiment 1 proton exchange membrane of the present invention
[0047] Weigh 30 grams of polyvinylidene fluoride and add it into 300 ml of tetrahydrofuran dissolved in sulfuric acid, the concentration of sulfuric acid is 3M, and keep stirring, raise the temperature to 70°C and keep it for 3 hours. After the polymer was completely dissolved, the solution was naturally cooled to room temperature for later use. 1.25 moles of vinyltrimethoxysilane were added to a mixture of 1 mole of polyethylene glycol monomethyl ether (molecular weight: 750) and 1.5 grams of catalyst (ammonium persulfate), and the reaction temperature was kept at 60 ° C. After 80 hours of reaction Finally, polyethylene glycol monomethyl ether terminated by a silane coupling agent was prepared for future use. A mixture of capped polyethylene glycol monomethyl ether and tetraethoxysilane (1:1 molar ratio) was used as nano-SiO 2 After mixing the precursors evenly, add them into 30...
Embodiment 2
[0048] Embodiment 2 The preparation (PFS) of proton exchange membrane of the present invention
[0049] Weigh 30 grams of polyvinylidene fluoride and add it into 250 ml of tetrahydrofuran dissolved in phosphoric acid, the concentration of phosphoric acid is 2M, and keep stirring, raise the temperature to 70°C and keep it for 3 hours. After the polymer was completely dissolved, the solution was naturally cooled to room temperature for later use. 1.25 moles of vinylmethyldimethoxysilane were added to a mixture of 1 mole of polyethylene glycol monomethyl ether (molecular weight: 750) and 1.5 grams of catalyst (ammonium persulfate), and the reaction temperature was maintained at 60 ° C during the reaction After 80 hours, polyethylene glycol monomethyl ether capped with a silane coupling agent was prepared for use. After mixing the end-capped polyethylene glycol monomethyl ether and tetraethoxysilane (the molar ratio is 3:7) as the precursor of nano-silica, add it to 200ml deioniz...
Embodiment 3
[0050] Embodiment 3 The preparation (SGS) of proton exchange membrane of the present invention
[0051] Weigh 25 g of polyvinyl alcohol and add it into 200 ml of aqueous solution containing sulfuric acid, the concentration of sulfuric acid is 3 M, and keep stirring, then raise the temperature to 90° C. and keep it for 3 hours. After the polymer is completely dissolved, the solution is naturally cooled to room temperature, and the polymer in the obtained solution is precipitated with acetone, a non-good solvent, to remove the reacted acid. After the obtained precipitate is dried to constant weight, it is dissolved in 300ml of A solution with a mass fraction of about 10% was obtained in ionized water for subsequent use. Add 1.25 moles of vinyltrimethoxysilane to a mixture of 1 mole of polyethylene glycol monomethyl ether (molecular weight is 750) and a certain amount of catalyst, and the reaction temperature is kept at 60°C. The polyethylene glycol monomethyl ether blocked by t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com