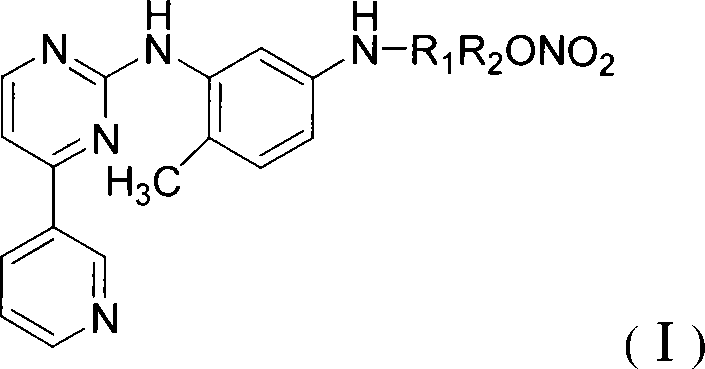
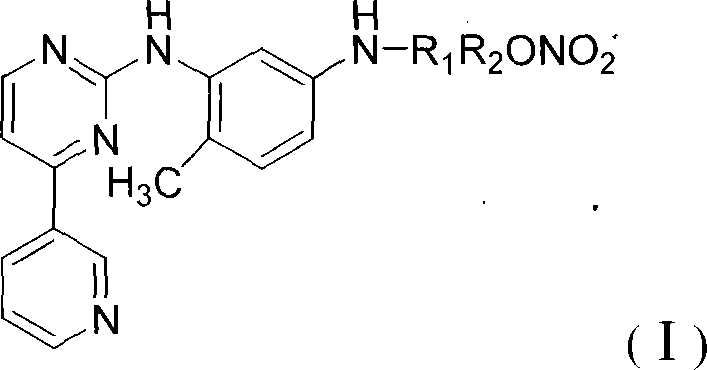
N-(5-amido-2-methyl phenyl)-4-(3-pyridinyl)-2-aminopyrimidine nitric oxide donating derivant, production method and uses thereof
A methyl and amino technology, applied in the field of N--4--2-pyrimidinamine derivatives, can solve the problems of short half-life, poor controllability, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
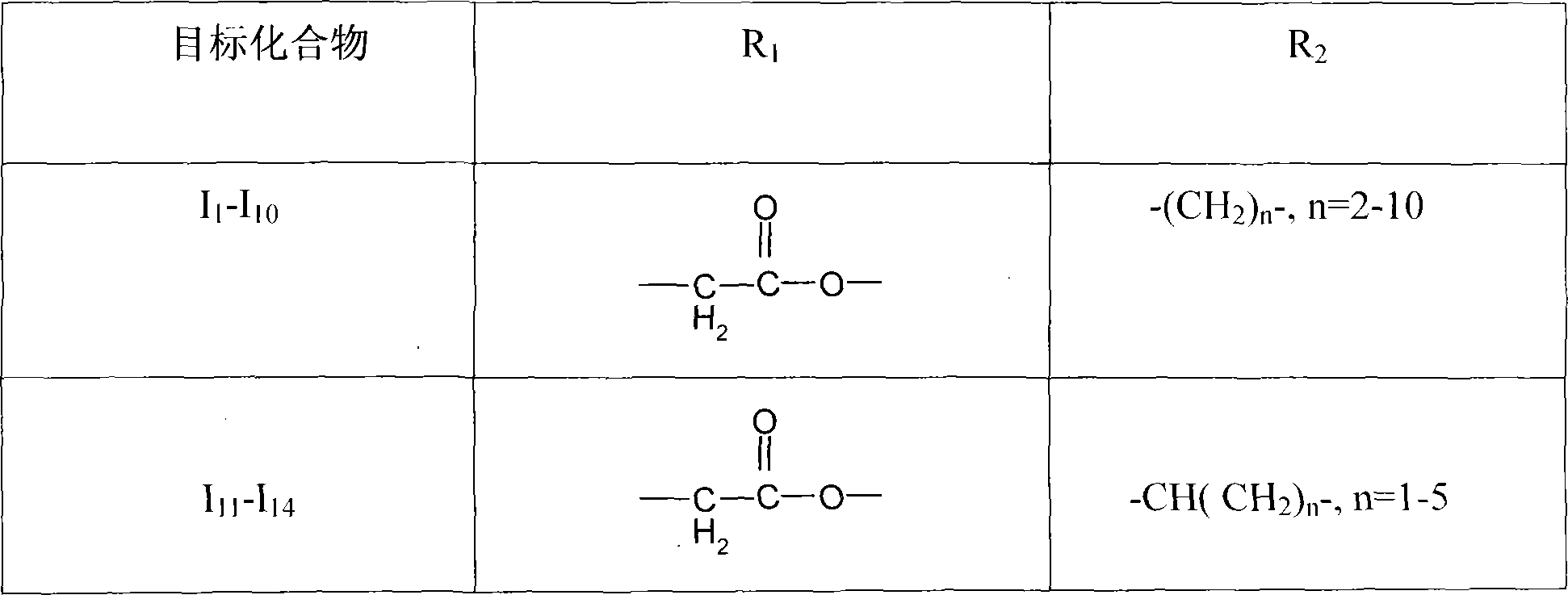
[0062] N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]-phenyl]acetic acid-2-nitrooxyethyl ester (I 1 )
[0063] 2-Nitroxyethanol
[0064] Under ice bath and nitrogen protection, ethylene glycol (2.48g, 40mmol) was dissolved in 100ml of ethyl acetate, and concentrated nitric acid (4.36g, 45mmol), 12ml of glacial acetic acid, and 10ml of acetic anhydride were added successively. Stir overnight at room temperature. Adjust the pH to neutral with 10% aqueous sodium bicarbonate solution, separate the organic layer, wash with saturated brine, dry over anhydrous potassium carbonate, and concentrate to obtain a yellow oily crude product. Column chromatography (ethyl acetate:petroleum ether=2:1) yielded 1.78g.
[0065] 2-Nitrooxyethyl bromoacetate
[0066] 2-Nitroxyethanol (1.42g, 13.27mmol) was dissolved in 40ml of anhydrous dichloromethane, bromoacetic acid (1.83g, 13.27mmol) was added under ice cooling, stirred and dissolved, DCC (2.74g, 13.30mmol) was added in batches ) an...
Embodiment 2
[0071] N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]-phenyl]acetic acid-6-nitrooxyhexyl ester (I 5 )
[0072] With reference to the preparation method of Example 1, N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]-phenyl]acetic acid is prepared from 1,6-hexanediol as starting material -6-Nitroxyhexyl ester, yield: 58.64%, mp: 123.07-124.8°C.
[0073] ESI-MS m / z: 481, 504 [M+Na] + ;
[0074] IR (KBr, v (cm -1 )): 3407(N-H), 3038(C-H, Ar), 1724(C=O), 1579(C=C), 1453(N-H), 1118(C-O-C), 1007(N-H)
[0075] 1 H-NMRδ (ppm): 9.3 (d, 1H, J = 1.81.NH), 8.7 (m, 2H), 8.4 (m, 2H,), 7.5 (dd, 1H, J1 = 4.76, J2 = 7.94), 7.4 (d, 1H, J = 5.13), 6.9 (d, 1H, J = 8.20), 6.8 (d, 1H, J = 2.07), 6.3 (dd, 1H, J = 210, J = 8.06), 5.87 ( s, 1H), 4.76(dd, 2H, J1=3.24, J2=5.40), 4.43(m, 8H), 3.94(d, 2H, J1=6.38), 2.1(s, 3H), 1.89(m, 2H )
Embodiment 3
[0077] N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]-phenyl]acetic acid-10-nitroxydecyl ester (I 9 )
[0078] Referring to the preparation method of Example 1, it is prepared from 1,10-decanediol as a starting material, N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]-phenyl] 10-Nitroxydecyl acetate, yield 53.43%, mp: 145.3-146.7°C.
[0079] ESI-MS m / z: 524[M+1] + , 546[M+Na] + ;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com