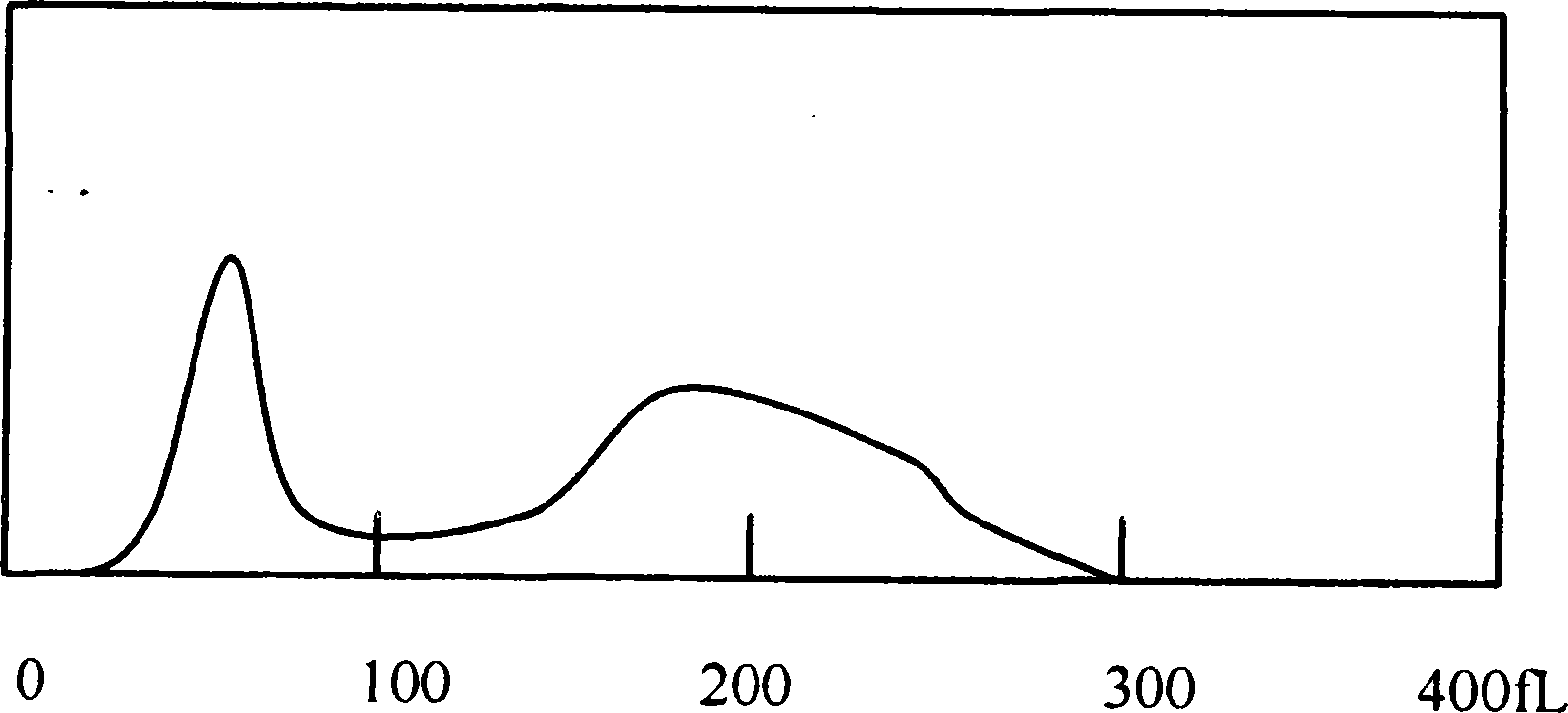
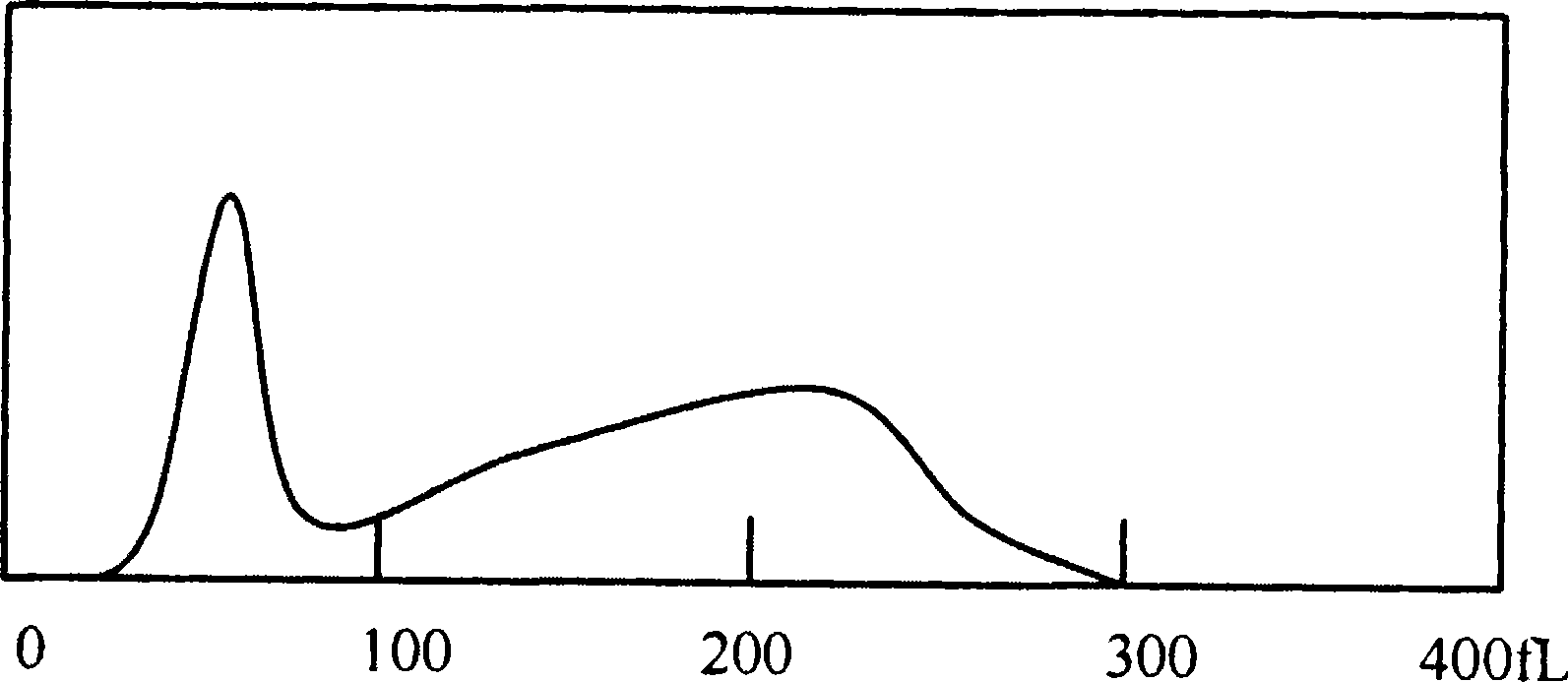
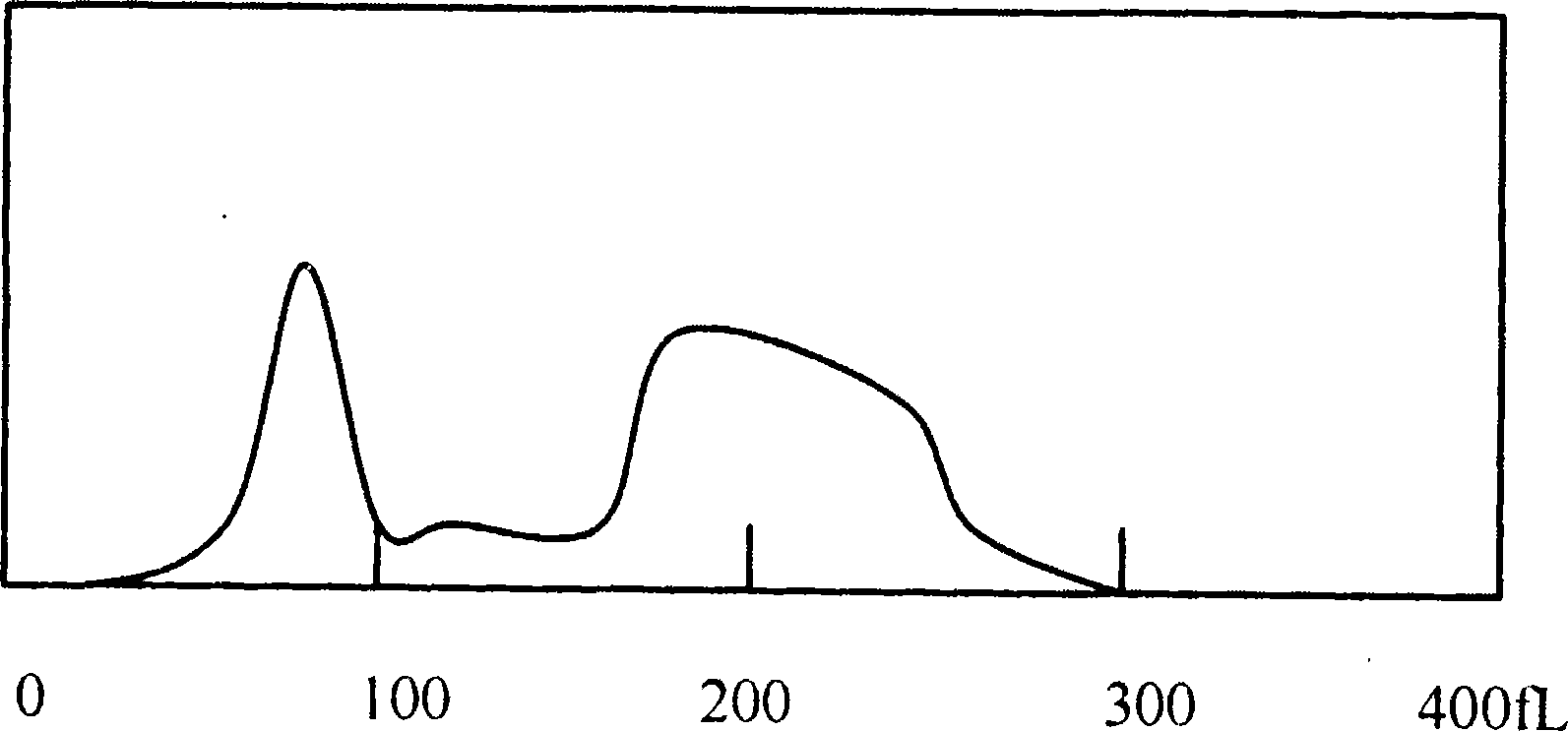
Hemolytic agent for measuring white blood cell in hemocyte
A hemolytic agent and white blood cell technology, which is applied in the field of medical reagents for blood cell detection, can solve the problems of low error and achieve the effect of low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Preparation of buffer solution [0.2mol / L phosphate buffer solution (pH 6.2)]: Weigh 8.82g of disodium hydrogen phosphate 12 water, dissolve it in deionized water and set the volume to 1L, and dilute it with sodium hydroxide and hydrochloric acid. The pH is adjusted so that the pH of the buffer is 6.2, and a sodium chloride regulator is added to make the osmotic pressure of the buffer 150mOsm / kg.
[0038] 2) a hemolytic agent for measuring white blood cells in blood cells, the preparation method of which is as follows:
[0039] Will C 12 h 25 -O-(CH 2 CH 2 O) 9 -H 16g and
[0040] Glutaraldehyde 1.0g and
[0041] Hexadecyltrimethylammonium Chloride C 16 h 33 (CH 3 ) 3 ClN 3.1g and
[0042] Buffer 14g
[0043] Set the volume to 1000ml with deionized water to obtain the hemolytic agent for the determination of leukocytes in blood cells.
[0044] The blood sample is detected in the blood cell analyzer by using the hemolyzing agent completed by the components...
Embodiment 2
[0049] 1) Preparation of buffer solution [0.2mol / L phosphate buffer solution (pH 6.3)]: Weigh 27.34g of sodium dihydrogen phosphate·2 water, dissolve with deionized water and dilute to 1L. The pH is adjusted with sodium hydroxide and hydrochloric acid to make the pH of the buffer solution 6.3, and a sodium chloride regulator is added to make the osmotic pressure of the buffer solution 250 mOsm / kg.
[0050] 2) a hemolytic agent for measuring white blood cells in blood cells, the preparation method of which is as follows:
[0051] Will C 12 h 25 -O-(CH 2 CH2 O) 9 -H 6.0g and
[0052] Formaldehyde 1.0g and
[0053] Hexadecyltrimethylammonium bromide C 16 h 33 (CH 3 ) 3 BrN 2.6g and
[0054] Buffer 11g
[0055] Set the volume to 1000ml with deionized water to obtain the hemolytic agent for the determination of leukocytes in blood cells.
Embodiment 3
[0057] 1) Preparation of buffer: [Borate buffer (pH9.1)]: Weigh 54.64g of sodium tetraborate with 10 crystal waters and dilute to 1000ml with deionized water. Adjust the pH with sodium hydroxide and hydrochloric acid to make the pH of the buffer solution 9.1, and add sodium sulfate regulator to make the osmotic pressure of the buffer solution 550 mOsm / kg.
[0058] 2) a hemolytic agent for measuring white blood cells in blood cells, the preparation method of which is as follows:
[0059] Will 22.0g and
[0060] Glutaraldehyde 6.0g and
[0061] Hexadecyltrimethylammonium Chloride C 16 h 33 (CH 3 ) 3 BrN 9.0g and
[0062] Buffer 16.0g
[0063] Set the volume to 1000ml with deionized water to obtain the hemolytic agent for the determination of leukocytes in blood cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com