Acetate doping natrium cobaltite thermoelectric materials and preparation method thereof
A thermoelectric material, acetate technology, applied in chemical instruments and methods, cobalt compounds, inorganic chemistry, etc., can solve the problems of long time, easy gel breakage, difficult to accurately grasp the drying process, etc., to achieve high chemical uniformity, Significant effect of layered structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
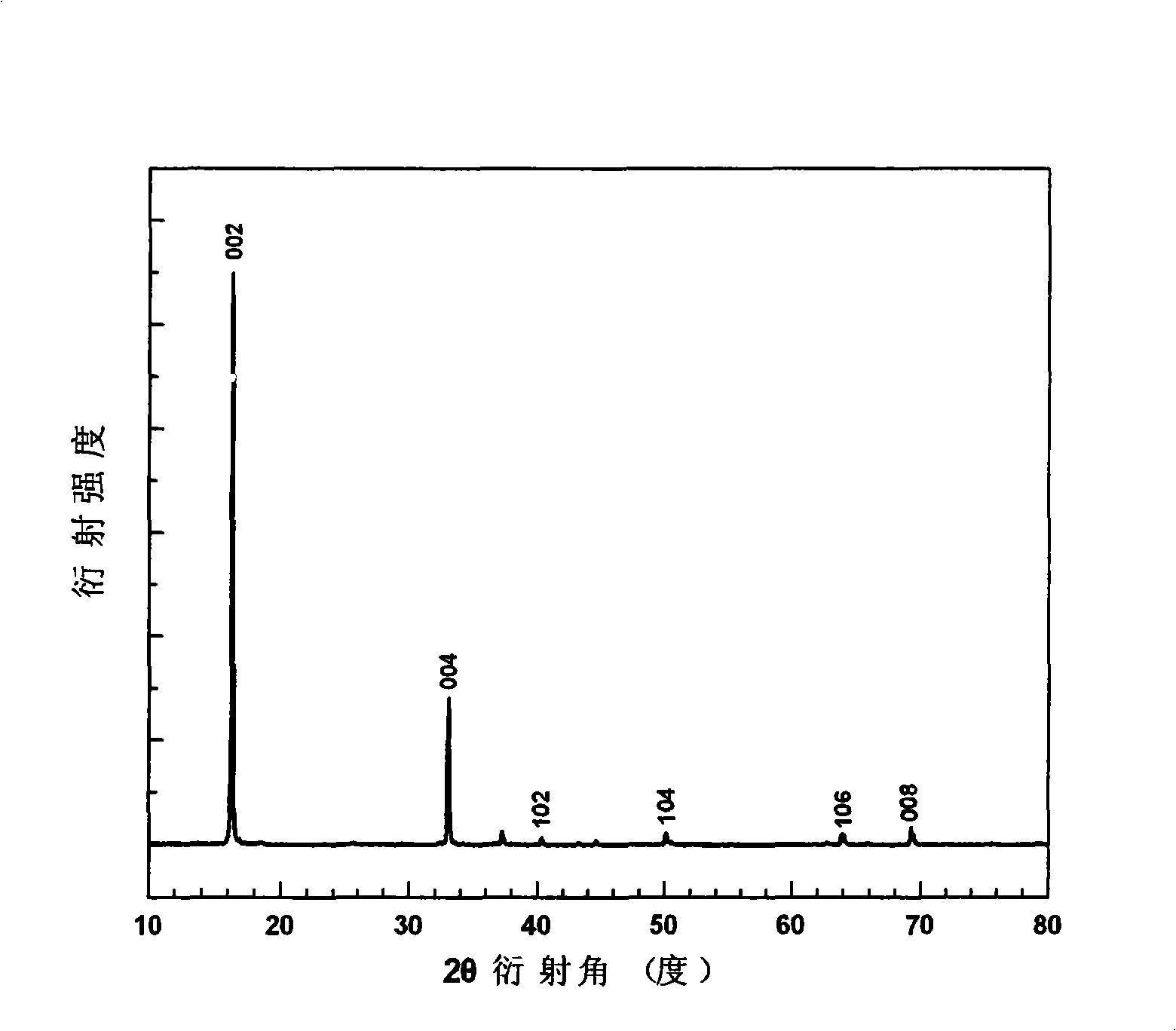
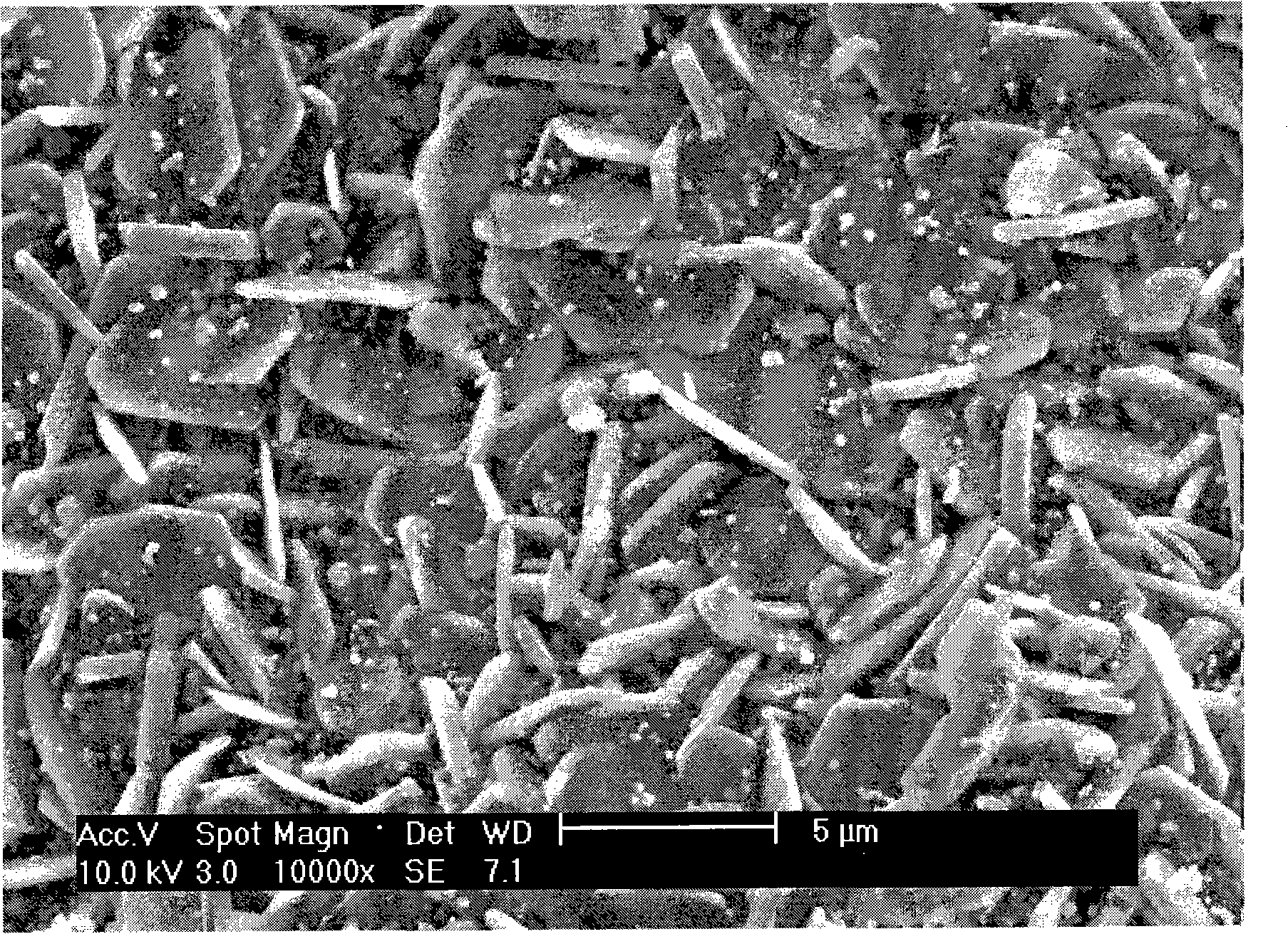
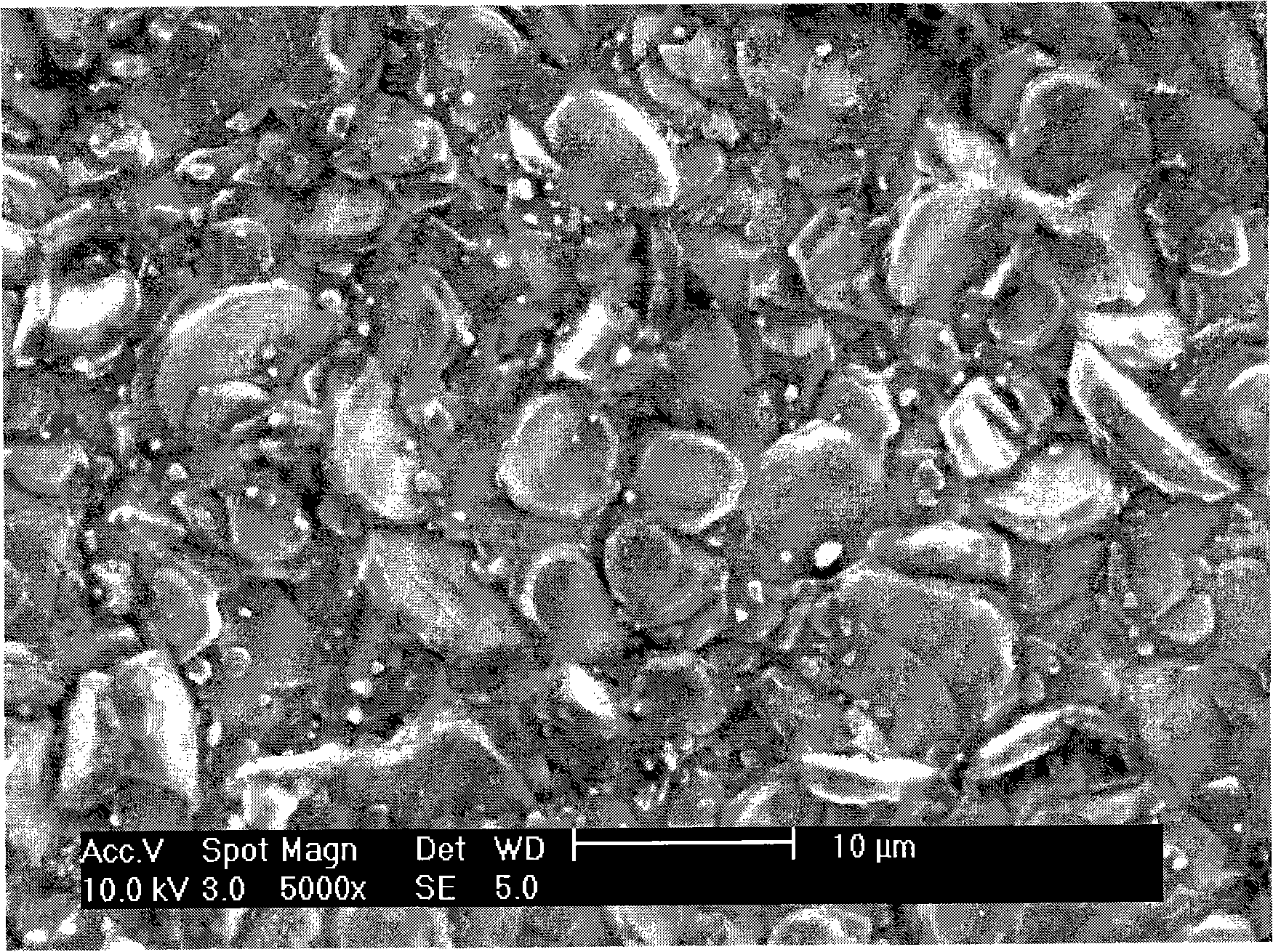
Image
Examples
Embodiment 1
[0058] Example 1. Na 1.5 (Co 0.9 Ni 0.1 ) 2 o 4
[0059] CH 3 COONa·3H 2 O (75mmol), (CH 3 COO) 2 Co 4H 2 O (90mmol), (CH 3 COO) 2 Ni·4H 2 O (10mmol), C 6 h 8 o 7 ·H 2 O (525mmol) was added to the beaker, and then the mixture was dissolved with 1600ml deionized water. Heat and stir at 323K to form a homogeneous solution. Pour it into a rotary evaporator, and evaporate under reduced pressure for 1 hour at a temperature of 353K and a pressure of less than 0.1MP, and the solution gradually becomes viscous. The viscous sol was placed in an oven, and dried under reduced pressure for 120 minutes at a constant temperature of 373K and a pressure of less than 0.1MP to form a fluffy xerogel. The xerogel was further pyrolyzed at 693K for 6 hours to fully drive off the residual organic matter and form a black precursor. After calcination at 1033K for 6 hours, the sintering precursor powder is formed. Grind the sintered precursor powder to a uniform powder, press moldin...
Embodiment 2
[0060] Example 2. Na 1.5 (Co 0.9 Cu 0.1 ) 2 o 4
[0061] CH 3 COONa·3H 2 O (75mmol), (CH 3 COO) 2 Co 4H 2 O (90mmol), (CH 3 COO) 2 Cu·H 2 O (10mmol), C 6 h 8 o 7 ·H 2 O (525mmol) was added to the beaker, and then the mixture was dissolved with 1600ml deionized water. Heat and stir at a temperature of 313K-333K to form a uniform solution. Pour it into a rotary evaporator, and evaporate under reduced pressure for 1 hour at a temperature of 353K-363K and a pressure of less than 0.1MP, and the solution gradually becomes viscous. The viscous sol is placed in an oven, and dried under reduced pressure for 110 minutes at a temperature of 323K-393K and a pressure of less than 0.1MP to form a fluffy xerogel. The xerogel was further pyrolyzed at 693K-773K for 7 hours to fully drive off residual organic matter and form a black precursor. After calcination at 993K-1053K for 6 hours, the sintering precursor powder is formed. Grind the sintered precursor powder to a unifo...
Embodiment 3
[0062] Example 3. Na 1.5 (Co 0.85 Cu 0.15 ) 2 o 4
[0063] CH 3 COONa·3H 2 O (75mmol), (CH 3 COO) 2 Co 4H 2 O (85mmol), (CH 3 COO) 2 Cu·H 2 O (15mmol), C 6 h 8 o 7 ·H 2 O (525mmol) was added to the beaker, and then the mixture was dissolved with 1600ml deionized water. Heat and stir at a temperature of 313K to form a homogeneous solution. Pour it into a rotary evaporator, and evaporate under reduced pressure for 1 hour at a temperature of 353K and a pressure of less than 0.1MP, and the solution gradually becomes viscous. The viscous sol was placed in an oven at a constant temperature of 323K and the pressure was less than 0.1MP, and dried under reduced pressure for 100 minutes to form a fluffy xerogel. The xerogel was further pyrolyzed at 773K for 6 hours to fully drive off the residual organic matter and form a black precursor. After calcination at 1053K for 6 hours, the sintering precursor powder is formed. Grind the sintered precursor powder to a uniform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thermoelectric figure of merit | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com