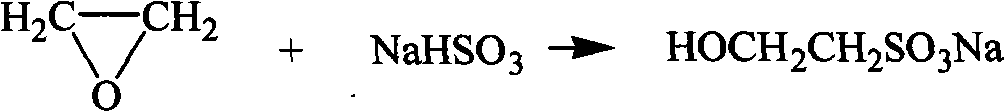Method for preparing taurine
A technology of taurine and isethionic acid, applied in the direction of organic chemistry, etc., can solve the problems of harsh production environment, excessive soda ash, decomposition of taurine, etc., to reduce power consumption and labor consumption, good production environment, The effect of improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
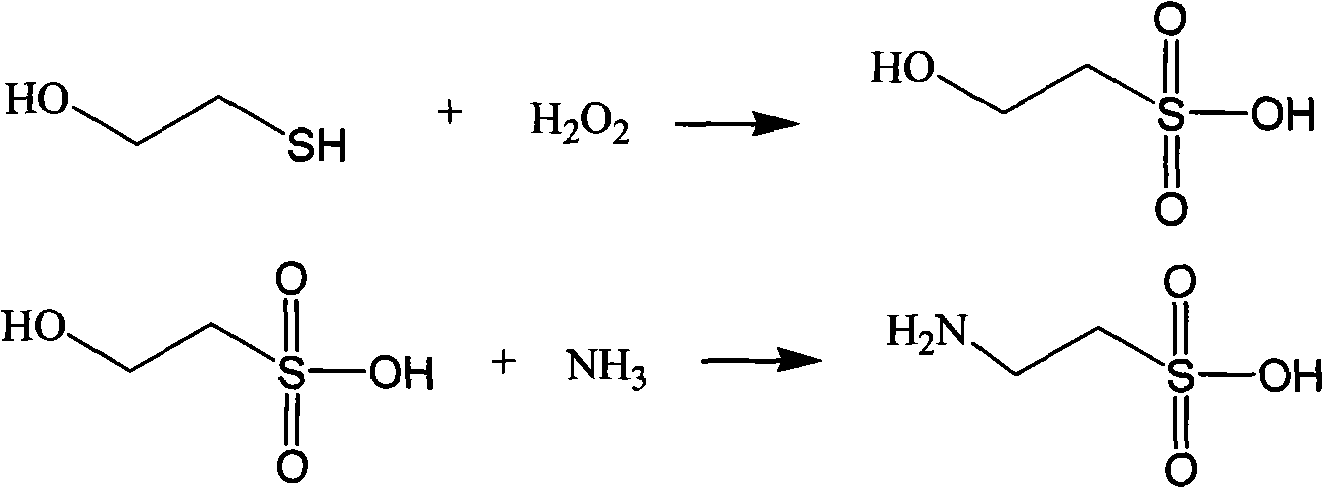
[0055] At 30° C., 78 g (1 mol) of hydroxyethyl mercaptan and 1133 g of 12% hydrogen peroxide (4 mol) were added to a 2000 mL autoclave, and the reaction was stirred for 3 hours after sealing. After the reaction, an isethionic acid solution was obtained.
[0056] Add 11mol ammonia to the isethionic acid solution obtained above (containing 1mol isethionic acid), add the solution to the pressure reactor, react at 260 degrees for 5 hours, drop to room temperature, and dropwise add hydrochloric acid to the precipitation. The solid was filtered and dried to obtain 100.2 g of taurine.
Embodiment 2
[0058] At 25°C, 78g (1mol) of hydroxyethyl mercaptan and 342g (4.5mol) of peracetic acid were added to a 2000mL autoclave, 1000mL of water was added, and the reaction was stirred for 6 hours after sealing. After the reaction, an isethionic acid solution was obtained.
[0059] Add 15mol ammonia to the isethionic acid solution obtained above (containing 1mol isethionic acid), add the solution to the pressure reactor, at 280 degrees, react for 3 hours, drop to room temperature, and dropwise add hydrochloric acid until it separates out. The solid was filtered and dried to obtain 105.6 g of taurine.
Embodiment 3
[0061] Under 20 ℃, in 2000mL autoclave, add the peroxybenzoic acid of 78g (1mol) hydroxyethyl mercaptan and 753g (5.5mol), add 700mL water and 50mL DMF, stir and react 8 hours after sealing, get hydroxyethyl alcohol after the reaction finishes. sulfonic acid solution.
[0062] Add 19mol ammonia to the isethionic acid solution obtained above (containing 1mol isethionic acid), add the solution to the pressure reactor, react at 300 degrees for 2 hours, drop to room temperature, and dropwise add hydrochloric acid to the precipitation. The solid was filtered and dried to obtain 101.2 g of taurine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com